Understanding the Complexity of Diabetes Mellitus Tipo 2
Diabetes mellitus tipo 2, often referred to as type 2 diabetes, represents one of the most complex and prevalent chronic conditions in global healthcare. Characterized by impaired glucose regulation, it is far more than a simple issue of elevated blood sugar. The underlying pathology involves a network of disrupted biological processes, including insulin resistance, beta-cell dysfunction, and chronic low-grade inflammation. While the disease is largely associated with lifestyle and genetic risk factors, its development is deeply rooted in intricate endocrine and metabolic imbalances.
You may also like: Breakthroughs in Current Diabetes Research: What the Latest Studies Reveal About Treatment and Prevention
Globally, diabetes mellitus tipo 2 continues to rise at an alarming rate. According to the International Diabetes Federation, over 500 million adults are currently living with diabetes, the vast majority of whom have type 2. The condition not only burdens individuals but also imposes staggering economic and healthcare costs. Understanding the pathophysiology of type 2 diabetes is therefore critical for advancing prevention, diagnosis, and treatment strategies.
What Characterizes Type 2 Diabetes at the Cellular Level
What characterizes type 2 diabetes is not merely elevated blood glucose but rather the fundamental breakdown in insulin signaling pathways and beta-cell function. In healthy individuals, insulin—a hormone secreted by pancreatic beta cells—facilitates the uptake of glucose into tissues such as muscle and fat. However, in those with diabetes mellitus tipo 2, these cells gradually become desensitized to insulin, a condition known as insulin resistance.
This resistance forces the pancreas to compensate by producing even more insulin. Over time, the pancreatic beta cells become dysfunctional and fail to meet the body’s growing insulin demands. This dual defect—insulin resistance and beta-cell failure—is central to the pathophysiology of dm type 2. Moreover, this metabolic imbalance triggers a cascade of events affecting multiple organ systems, contributing to the progressive nature of the disease.
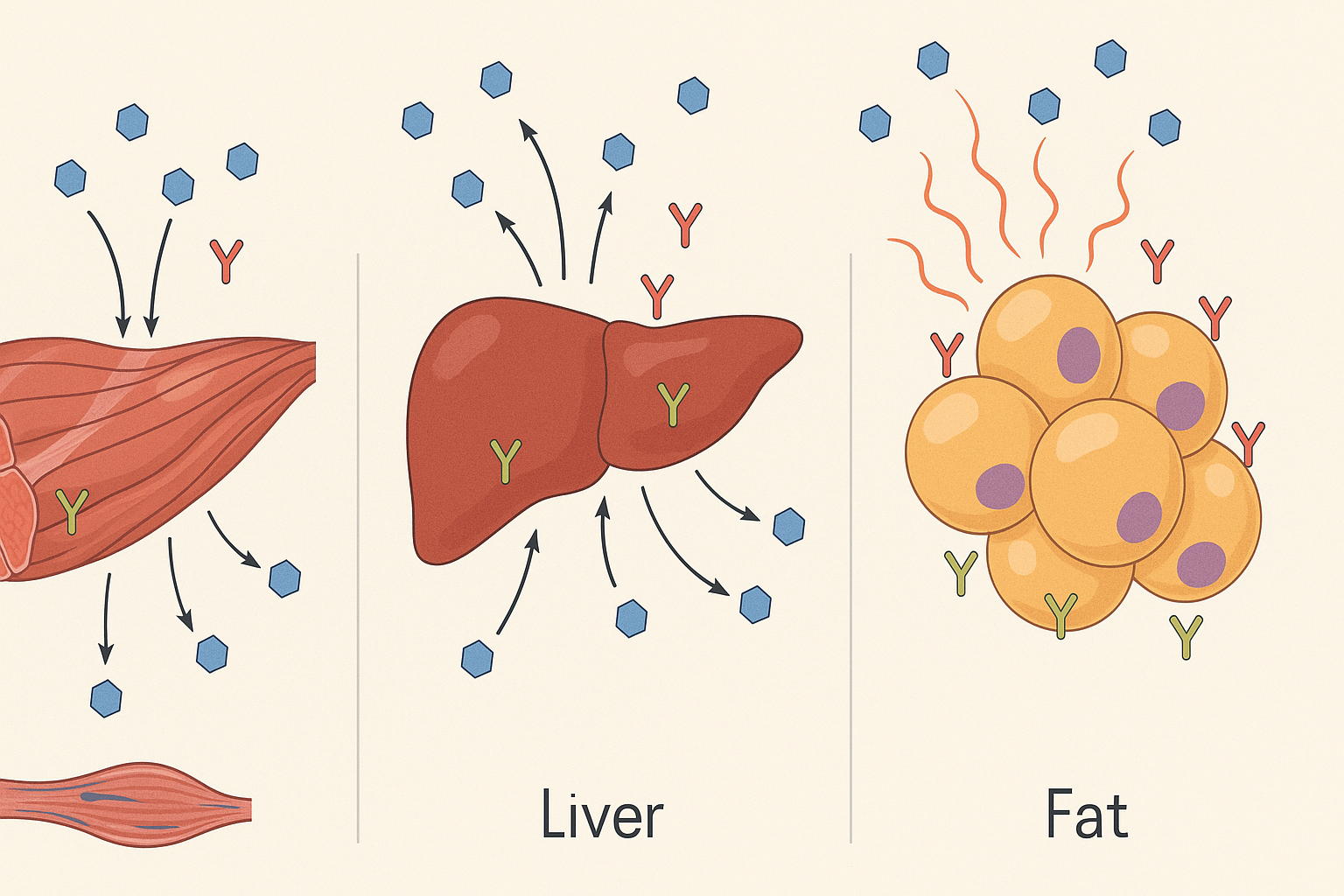
Exploring the Pathophysiology of Type 2 Diabetes
The pathophysiology of type 2 diabetes involves a combination of genetic predisposition and environmental triggers. Insulin resistance typically emerges first, often years before any clinical diagnosis. This resistance impairs the normal physiological response to insulin in peripheral tissues, especially skeletal muscle, liver, and adipose tissue. The liver, for instance, continues to produce glucose even in the presence of high insulin levels, exacerbating hyperglycemia.
Simultaneously, the beta cells of the pancreas attempt to compensate by secreting additional insulin. Initially, this compensatory hyperinsulinemia maintains relatively normal glucose levels. However, over time, the chronic demand for insulin leads to beta-cell exhaustion, loss of function, and even apoptosis. This failure of insulin production is a hallmark feature of the pathophysiology of type ii diabetes and marks the transition from insulin resistance to full-blown hyperglycemia.
Inflammation also plays a pivotal role in the patho of type 2 diabetes. Adipose tissue, particularly visceral fat, becomes a source of pro-inflammatory cytokines such as TNF-alpha and interleukin-6. These molecules interfere with insulin signaling and exacerbate insulin resistance. The interplay between immune cells, adipokines, and insulin receptors is now recognized as a crucial element in the progression of the disease.
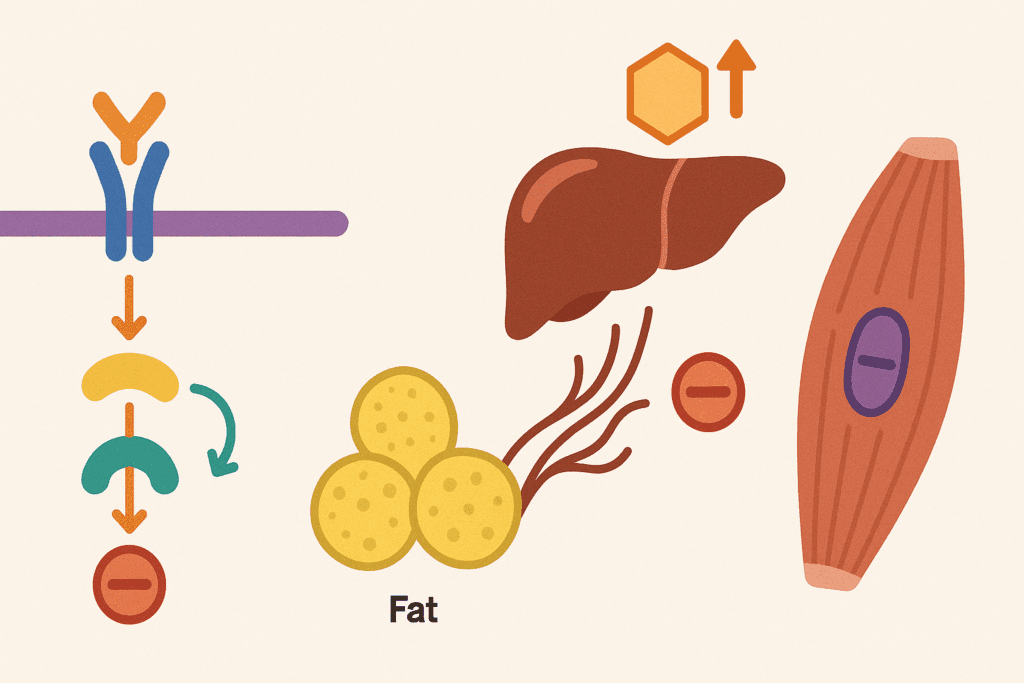
Decoding the Type 2 Diabetes Mellitus Mechanism
To fully understand the type 2 diabetes mellitus mechanism, it is essential to consider the hormonal and cellular disruptions occurring in tandem. Insulin resistance is often the initiating defect. Muscle cells fail to respond adequately to insulin, resulting in decreased glucose uptake. The liver contributes by overproducing glucose, and the fat cells release excess free fatty acids, which further inhibit insulin action.
On a molecular level, defects in the insulin receptor substrate (IRS) signaling cascade impair glucose transporter (GLUT4) translocation, particularly in muscle tissue. The failure of GLUT4 to mobilize to the cell membrane directly leads to decreased glucose uptake and utilization. These biochemical failures lie at the core of the type 2 diabetes mechanism and highlight the multifactorial nature of the disorder.
Another vital element is the role of incretin hormones such as GLP-1, which are reduced in individuals with type 2 diabetes mellitus. Incretins normally enhance insulin secretion after meals and suppress glucagon. Their dysfunction contributes to postprandial hyperglycemia and reduced insulin effectiveness, further compounding the metabolic derangements.
The Onset of Diabetes That Occurs Later in Life
One defining feature of diabetes mellitus tipo 2 is its gradual onset. Often described as the onset of diabetes that occurs later in life, this form of the disease typically manifests after the age of 40, although it is increasingly being diagnosed in younger individuals due to rising rates of obesity and sedentary lifestyles. Unlike type 1 diabetes, which can present abruptly, the progression of type 2 diabetes is insidious and may go undetected for years.
This delayed onset complicates early diagnosis and intervention. Many individuals remain asymptomatic until complications have already begun to develop. The subtle nature of its early symptoms—such as fatigue, frequent urination, and blurry vision—are often attributed to aging or other benign causes. This contributes to the high proportion of undiagnosed cases globally and underscores the importance of proactive screening and education.
Additionally, the age-related decline in pancreatic beta-cell function and increasing insulin resistance with advancing age further compound the risk. These age-associated physiological changes reinforce the need for age-specific strategies in screening and managing type 2 diabetes mellitus.
Clarifying the Diabetes Abbreviation and Clinical Terminology
In clinical practice, the diabetes abbreviation for type 2 diabetes is commonly written as T2DM, short for type 2 diabetes mellitus. Other shorthand notations include DM2 or simply DM. These abbreviations are widely used in medical records, research literature, and patient education materials. Understanding these terms is crucial for navigating clinical documentation and communication between healthcare professionals.
The diabetes mellitus medical abbreviation not only simplifies communication but also distinguishes between different types of diabetes. For instance, type 1 diabetes is often abbreviated as T1DM, while gestational diabetes may be labeled GDM. The abbreviation for diabetes type 2 helps ensure clarity, particularly when discussing complications, treatment options, or research findings.
Healthcare professionals should take care to explain these abbreviations to patients during consultations. While these terms may be second nature to clinicians, they can be confusing for patients unfamiliar with medical jargon. Empowering patients with this knowledge enhances health literacy and facilitates more effective shared decision-making.
Unraveling the Pathophysiology of DM2 and Its Clinical Significance
The pathophysiology of dm2 continues to evolve as research uncovers new insights into its molecular and systemic effects. It is increasingly evident that type 2 diabetes is not a homogeneous disease but rather a spectrum of disorders involving variable degrees of insulin resistance, beta-cell dysfunction, and organ-specific complications.
Emerging research has highlighted the role of mitochondrial dysfunction in the development of insulin resistance. Mitochondria are central to energy metabolism, and their impairment contributes to oxidative stress, inflammation, and disrupted glucose homeostasis. This mitochondrial link adds another layer to the pathophysiology of type 2 diabetes and opens new avenues for therapeutic intervention.
Epigenetic modifications have also been implicated in the development of diabetes mellitus tipo 2. Environmental factors such as diet and physical activity can influence gene expression through mechanisms like DNA methylation and histone modification. These changes can alter insulin sensitivity and beta-cell resilience, providing a molecular explanation for how lifestyle factors contribute to disease risk.
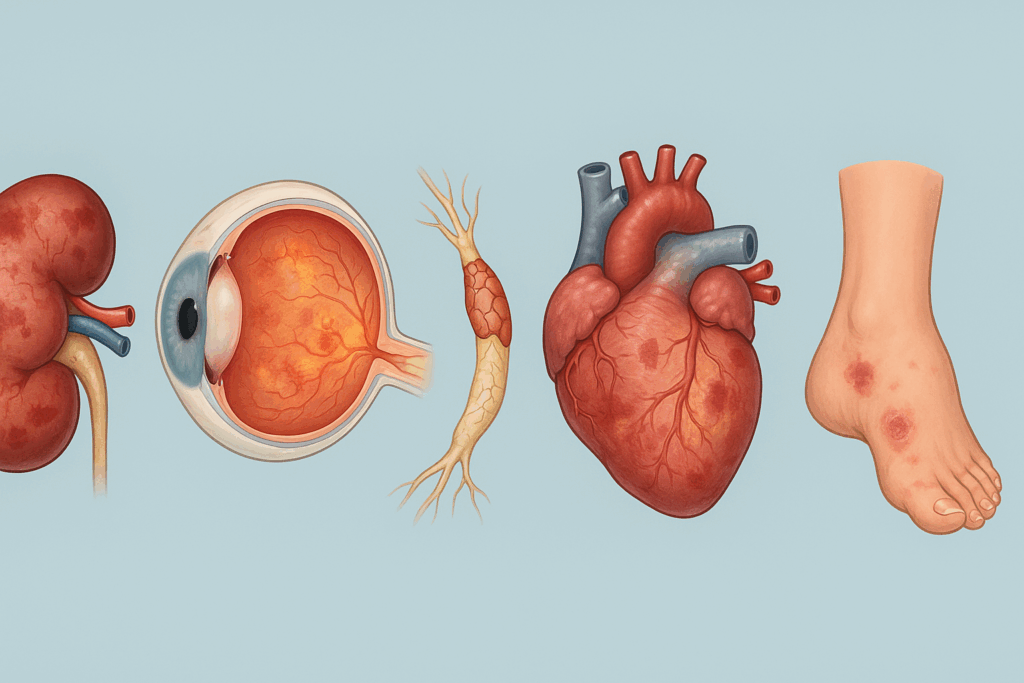
Complications Associated with Type 2 Diabetes Mellitus
The phrase “type 2 diabetes mellitus with complication” refers to the many ways in which prolonged hyperglycemia affects multiple organ systems. Chronic exposure to elevated blood glucose leads to damage in both the microvascular and macrovascular systems. Microvascular complications include retinopathy, nephropathy, and neuropathy, while macrovascular issues encompass coronary artery disease, stroke, and peripheral artery disease.
Diabetic nephropathy, characterized by progressive kidney damage, is a leading cause of end-stage renal disease. Retinopathy, resulting from damage to the small vessels in the retina, remains a major cause of preventable blindness. Meanwhile, diabetic neuropathy can lead to chronic pain, loss of sensation, and increased risk of foot ulcers and amputations.
The presence of type 2 diabetes mellitus with complication significantly worsens prognosis and quality of life. Early detection and aggressive management of blood glucose, blood pressure, and lipid levels are essential to preventing or delaying these outcomes. Integrated care models that emphasize multidisciplinary collaboration have shown promise in reducing complication rates and improving patient outcomes.
What Is the General Pathology Associated with Type 2 Diabetes?
At a broader level, what is the general pathology associated with type 2 diabetes involves systemic metabolic dysfunction. Hyperglycemia acts as both a symptom and a cause of further metabolic derangement. Chronic glucose elevation promotes the formation of advanced glycation end-products (AGEs), which damage tissues and accelerate the aging process.
Endothelial dysfunction, inflammation, and oxidative stress become prominent features of the disease. These processes not only impair vascular function but also alter immune responses, making individuals more susceptible to infections and delayed wound healing. Dyslipidemia—another common feature of type 2 diabetes—adds to cardiovascular risk by promoting atherogenesis.
Understanding this broader pathology is critical for holistic treatment strategies. Management should extend beyond glucose control to encompass cardiovascular risk reduction, renal protection, and neurological health. Comprehensive care plans that address these multifaceted issues can significantly reduce the long-term burden of diabetes mellitus tipo 2.
Looking Ahead: Therapeutic Innovations Targeting the Pathophysiology of DM Type 2
Recent advancements in pharmacology and biotechnology are shedding new light on how we approach the pathophysiology of dm type 2. Drugs targeting specific molecular pathways—such as SGLT2 inhibitors and GLP-1 receptor agonists—have shown benefits that extend beyond glycemic control. These therapies not only improve insulin sensitivity but also offer cardiovascular and renal protection.
Gene therapy and stem cell research are also being explored as potential treatments. These approaches aim to restore beta-cell function or regenerate damaged pancreatic tissue. While still in experimental stages, they hold immense promise for altering the course of type 2 diabetes mellitus.
Lifestyle interventions remain foundational to managing the disease. Diet, physical activity, and behavioral counseling have demonstrated effectiveness in both preventing and treating type 2 diabetes. However, understanding the type 2 diabetes mellitus mechanism allows for more targeted and individualized interventions that address each patient’s unique pathophysiological profile.
Frequently Asked Questions: Advanced Insights into Type 2 Diabetes and Its Mechanisms
What are some lesser-known early signs of the onset of diabetes that occurs later in life?
While classic symptoms like frequent urination and fatigue are widely recognized, subtler indicators often precede the clinical diagnosis of diabetes mellitus tipo 2. These may include increased skin infections, prolonged healing of minor wounds, and subtle vision changes even before retinopathy is detectable. Some individuals experience mood disturbances such as irritability or cognitive sluggishness due to fluctuating blood glucose. Another overlooked sign during the onset of diabetes that occurs later in life is altered sleep patterns, which may stem from nighttime polyuria or unrecognized neuropathy. Recognizing these early red flags, especially when there’s a family history, allows for timely intervention and lifestyle modifications that may delay progression.
How does chronic stress influence the pathophysiology of type 2 diabetes?
Chronic psychological stress activates the hypothalamic-pituitary-adrenal (HPA) axis, leading to sustained elevation of cortisol levels. Cortisol directly impairs insulin sensitivity and promotes hepatic glucose production, thereby worsening the pathophysiology of dm type 2. Additionally, stress contributes to poor sleep and dietary choices, which further disrupt glucose metabolism. Stress-induced inflammation also plays a central role in the patho of type 2 diabetes, as it perpetuates insulin resistance via cytokine production. Long-term stress management techniques—such as mindfulness, cognitive behavioral therapy, and physical activity—can be valuable adjuncts to traditional pharmacological treatment.
Can the type 2 diabetes mellitus mechanism be influenced by gut microbiota?
Yes, recent research has uncovered that gut microbiota composition plays a significant role in modulating insulin sensitivity and inflammation. Certain bacterial strains promote the production of short-chain fatty acids (SCFAs), which improve glucose uptake and reduce systemic inflammation, thereby influencing the type 2 diabetes mechanism. Dysbiosis, or microbial imbalance, can increase gut permeability and lead to endotoxemia, which exacerbates the pathophysiology of type ii diabetes. Emerging therapies targeting the microbiome—such as prebiotics, probiotics, and dietary fiber—are under investigation for their potential to complement current treatments. These findings suggest that the gut may represent a modifiable factor in the pathophysiology of dm2.
What is the relevance of the diabetes abbreviation in clinical research and patient care?
The diabetes abbreviation serves more than just a shorthand function; it streamlines communication in electronic medical records and research protocols. Terms like T2DM or the dm 2 medical abbreviation help clinicians quickly distinguish between diabetes types and guide treatment algorithms accordingly. In the research setting, consistent use of the abbreviation for diabetes type 2 ensures clarity when aggregating large datasets or conducting meta-analyses. Patients, however, may find these abbreviations confusing without explanation. Enhancing patient education around the diabetes mellitus medical abbreviation can bridge the communication gap and foster better engagement with care plans.
How does type two diabetes endocrinology differ from that of other chronic endocrine disorders?
Type two diabetes endocrinology uniquely involves the dysregulation of multiple hormone systems, not solely insulin. Incretins, glucagon, leptin, and adiponectin all contribute to the hormonal landscape that shapes the pathophysiology of dm2. Unlike hypothyroidism or adrenal insufficiency, which often stem from single hormone deficits, type 2 diabetes mellitus mechanism reflects a dynamic interplay between hormone excess and resistance. Furthermore, endocrine adaptations in diabetes mellitus tipo 2 evolve over time, requiring longitudinal reassessment and often therapeutic adjustments. Understanding these nuances is key to optimizing care and anticipating complications.
What characterizes type 2 diabetes progression despite standard glucose-lowering therapy?
Many patients experience a gradual decline in glycemic control despite adherence to medication, raising questions about what characterizes type 2 diabetes beyond insulin resistance. Progressive beta-cell failure is central to this issue, compounded by glucotoxicity and lipotoxicity that further impair cellular function. Additionally, genetic polymorphisms and epigenetic influences can modify how individuals respond to medications, complicating the pathophysiology of type 2 diabetes. Even with ideal lifestyle management, factors such as aging and comorbidities accelerate disease progression. These complexities underscore the importance of individualized care plans that evolve in parallel with the disease.
How do environmental toxins contribute to the pathophysiology of dm type 2?
Emerging studies show that environmental exposures—including persistent organic pollutants and heavy metals—can disrupt endocrine function and insulin signaling. These toxins can mimic hormones or interfere with receptor pathways, thereby intensifying the type 2 diabetes mechanism. Chronic exposure may contribute to low-grade inflammation, oxidative stress, and mitochondrial dysfunction, key drivers in the patho of type 2 diabetes. Additionally, socioeconomic factors often influence exposure risk, making environmental justice a relevant concern in diabetes prevention. Detoxification strategies, regulatory policy changes, and further research are crucial to mitigate this underrecognized risk factor.
What is the general pathology associated with type 2 diabetes in the brain and nervous system?
While vascular complications are well documented, less attention is paid to how type 2 diabetes mellitus with complication affects the central nervous system. Hyperglycemia and insulin resistance are now linked to cognitive decline, reduced hippocampal volume, and increased Alzheimer’s disease risk. Neuroinflammation, driven by the pathophysiology of type ii diabetes, disrupts synaptic plasticity and impairs memory consolidation. Peripheral neuropathies are more common, but central effects deserve greater emphasis in both research and clinical practice. This neurological dimension expands what is the general pathology associated with type 2 diabetes and highlights the need for cognitive screening in long-term care.
Why are older adults at unique risk for underdiagnosis during the onset of diabetes that occurs later in life?
Older adults may attribute early symptoms to normal aging, which masks the onset of diabetes that occurs later in life. Additionally, polypharmacy and comorbid conditions can blur diagnostic clarity. Age-related physiological changes, such as diminished thirst perception and altered glucose metabolism, modify how diabetes mellitus tipo 2 presents in seniors. Social isolation and reduced healthcare access further delay diagnosis and treatment. A heightened clinical suspicion, coupled with routine screening for at-risk older adults, is essential to capture these often-overlooked cases.
How is the pathophysiology of dm2 evolving with the rise of personalized medicine?
Advances in genomics and metabolic profiling are transforming our understanding of the pathophysiology of dm type 2. By identifying genetic variants and molecular signatures, clinicians can stratify patients according to their risk, expected disease course, and likely response to therapy. This granular view moves beyond one-size-fits-all models and supports the development of precision interventions that align with an individual’s unique type 2 diabetes mellitus mechanism. Furthermore, digital health tools now allow continuous glucose and lifestyle monitoring, offering real-time feedback and personalization. As personalized medicine matures, it holds promise for altering the trajectory of diabetes mellitus tipo 2 and redefining therapeutic success.
Conclusion: Redefining Our Understanding of Type 2 Diabetes Through Science
As our knowledge deepens, it becomes increasingly clear that type 2 diabetes is a dynamic, multifactorial condition requiring nuanced understanding and individualized care. The pathophysiology of type 2 diabetes encompasses far more than insulin resistance and beta-cell failure; it is a reflection of systemic metabolic imbalance involving hormonal disruption, inflammation, mitochondrial dysfunction, and genetic susceptibility.
Clarifying the diabetes abbreviation and its underlying mechanisms aids both clinicians and patients in making informed decisions. From the early onset of diabetes that occurs later in life to the numerous complications that may follow, a comprehensive grasp of the patho of type 2 diabetes is vital for effective management and prevention.
By focusing on what characterizes type 2 diabetes at the cellular and systemic levels, healthcare professionals and researchers can better target interventions. As emerging therapies continue to evolve, the integration of scientific discovery with clinical practice offers renewed hope for reducing the global burden of diabetes mellitus tipo 2. Through continued investment in research and public education, we move closer to unraveling the full complexity of this modern epidemic and redefining its future.
Was this article helpful? Don’t let it stop with you. Share it right now with someone who needs to see it—whether it’s a friend, a colleague, or your whole network. And if staying ahead on this topic matters to you, subscribe to this publication for the most up-to-date information. You’ll get the latest insights delivered straight to you—no searching, no missing out.
Further Reading:
Research progress in the relationship between type 2 diabetes mellitus and intestinal flora
Genetics of Type 2 Diabetes—Pitfalls and Possibilities
Trailblazing Discoveries: The Top 5 Diabetes Research Breakthroughs of 2023
Disclaimer
The information contained in this article is provided for general informational purposes only and is not intended to serve as medical, legal, or professional advice. While MedNewsPedia strives to present accurate, up-to-date, and reliable content, no warranty or guarantee, expressed or implied, is made regarding the completeness, accuracy, or adequacy of the information provided. Readers are strongly advised to seek the guidance of a qualified healthcare provider or other relevant professionals before acting on any information contained in this article. MedNewsPedia, its authors, editors, and contributors expressly disclaim any liability for any damages, losses, or consequences arising directly or indirectly from the use, interpretation, or reliance on any information presented herein. The views and opinions expressed in this article are those of the author(s) and do not necessarily reflect the official policies or positions of MedNewsPedia.