Proteins are the building blocks of life, yet few people outside the field of biology understand how they are actually made in the body or why this process is vital to human health. Every muscle contraction, enzyme function, hormone signal, and cell structure depends on proteins. Understanding where proteins are made and how the body makes proteins is not only essential for those studying human physiology but also for anyone seeking to optimize their health through nutrition and wellness. This article offers an in-depth exploration of protein synthesis, tracing the intricate pathways of cellular machinery, dietary influences, and the far-reaching implications this process has on overall health.
You may also like: Macronutrients vs Micronutrients: What the Simple Definition of Macronutrients Reveals About Your Diet and Health
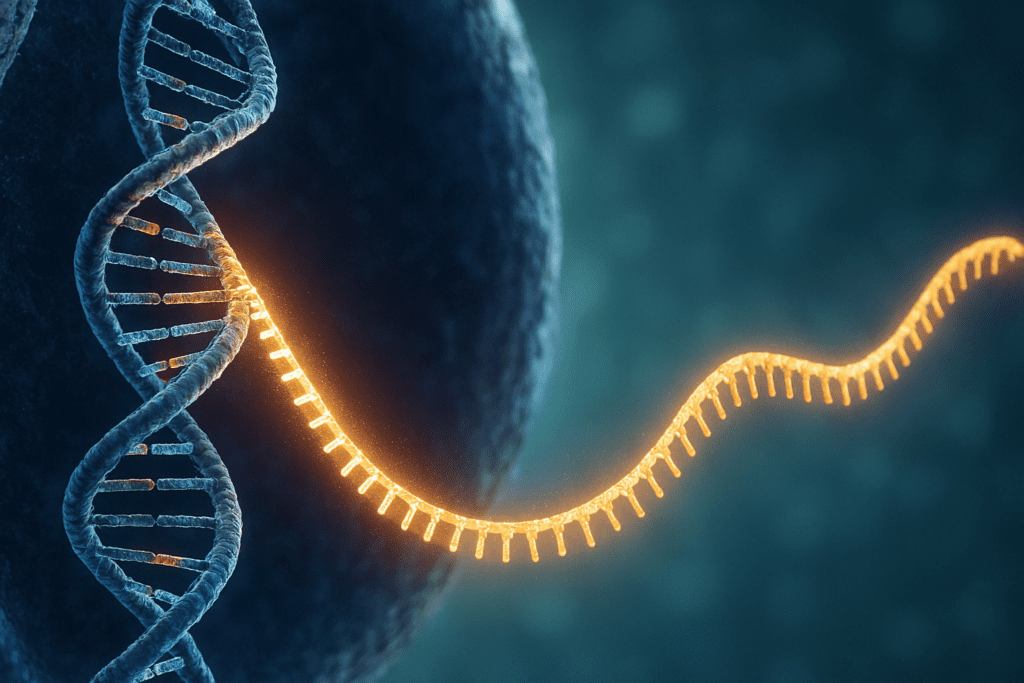
The Molecular Blueprint: DNA as the Instruction Manual for Making Proteins
The journey of protein synthesis begins in the nucleus of the cell, where DNA resides as the master blueprint. DNA contains the genetic instructions for building proteins in the form of sequences known as genes. Each gene corresponds to a specific protein or part of a protein, encoding the order of amino acids that must be assembled. However, DNA cannot leave the nucleus, so a critical intermediary is needed. This is where messenger RNA (mRNA) comes into play. Through a process called transcription, a portion of DNA is copied into an mRNA strand. This step sets the stage for where proteins are made, because the mRNA travels out of the nucleus and into the cytoplasm, where ribosomes take over.
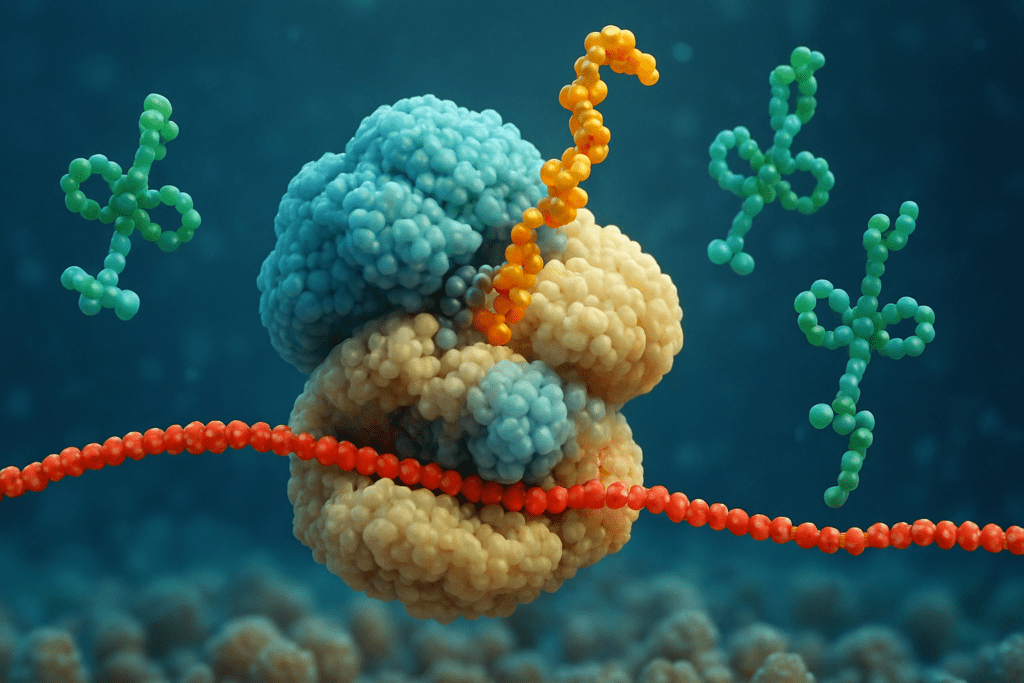
The Ribosome: The Central Hub Where Proteins Are Made
The ribosome is the cellular structure that actually makes proteins by assembling amino acids in the order dictated by the mRNA sequence. These tiny but powerful molecular machines float freely in the cytoplasm or attach themselves to the endoplasmic reticulum, forming what is known as the rough ER. This is the main site where proteins are made in cells. Once mRNA reaches a ribosome, translation begins. During this process, transfer RNA (tRNA) molecules bring specific amino acids to the ribosome, matching them to the codons on the mRNA template. This step-by-step decoding results in the formation of a polypeptide chain, which eventually folds into a functional protein. The sophistication of the ribosome’s operation cannot be overstated, and it exemplifies the complex biological choreography that enables life.
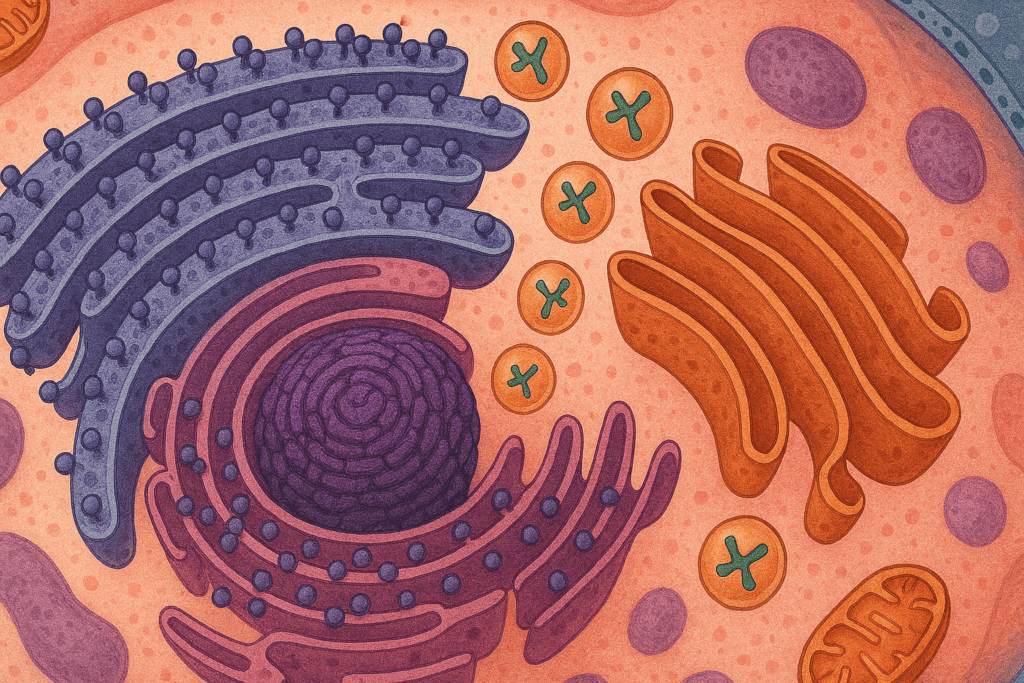
Endoplasmic Reticulum and Golgi Apparatus: Fine-Tuning the Final Product
While ribosomes are where proteins are made, the process does not end there. Many proteins undergo further modification and processing in other cellular structures. The endoplasmic reticulum (ER), especially the rough ER, is involved in folding and preliminary modifications. Proteins destined for secretion or membrane integration are guided into the ER, where they may be glycosylated, folded, or checked for errors. Once processed, these proteins are sent to the Golgi apparatus. Here, additional modifications, packaging, and sorting take place, ensuring that each protein reaches its intended destination within or outside the cell. These steps are crucial, as improperly folded or misdirected proteins can lead to serious health consequences, including neurodegenerative diseases and immune disorders.
Amino Acids: The Raw Materials for Protein Construction
To make proteins, the body must have access to amino acids—the raw materials used to build polypeptide chains. There are 20 standard amino acids, nine of which are considered essential because the body cannot produce them and must obtain them through diet. Protein-rich foods such as eggs, meat, fish, legumes, and dairy provide these critical building blocks. Without an adequate and diverse intake of amino acids, even the most efficient protein synthesis machinery cannot function properly. Nutritional deficiencies can impair the ability of the body to make proteins, leading to muscle wasting, immune dysfunction, and delayed wound healing. Therefore, understanding where proteins are made goes hand-in-hand with knowing how to fuel the process through proper dietary choices.
Nutritional Influence on Protein Synthesis
Nutrition plays a foundational role in the efficiency and regulation of protein synthesis. Macronutrient balance, meal timing, and micronutrient availability all influence how well the body makes proteins. For example, leucine—an essential amino acid found in high-protein foods—has been shown to directly stimulate the mTOR pathway, a key regulator of muscle protein synthesis. Similarly, deficiencies in vitamins and minerals such as zinc, magnesium, and B vitamins can compromise enzymatic functions essential to the transcription and translation processes. Athletes, older adults, and those recovering from surgery have especially high demands for protein synthesis, and their nutritional strategies often include increased protein intake and supplementation to support cellular repair and regeneration. These examples underscore that nutrition is not just a source of energy—it is a critical determinant of where and how efficiently proteins are made.
Protein Synthesis in Special Populations
Different physiological states influence the body’s capacity to make proteins. In growing children and adolescents, protein synthesis is elevated to support rapid cell proliferation and tissue development. Pregnant individuals also experience increased protein demands, both to support maternal health and to build fetal tissues. On the opposite end of the spectrum, aging often brings a decline in the body’s ability to make proteins efficiently. This is partly due to anabolic resistance—a diminished sensitivity to dietary amino acids that results in decreased muscle protein synthesis. Strategies such as resistance training and higher leucine intake have been shown to counteract this effect. Moreover, individuals with chronic illnesses or metabolic disorders may experience disruptions in protein metabolism, making it even more vital to understand the mechanisms of synthesis and the role nutrition plays in supporting or impairing these pathways.
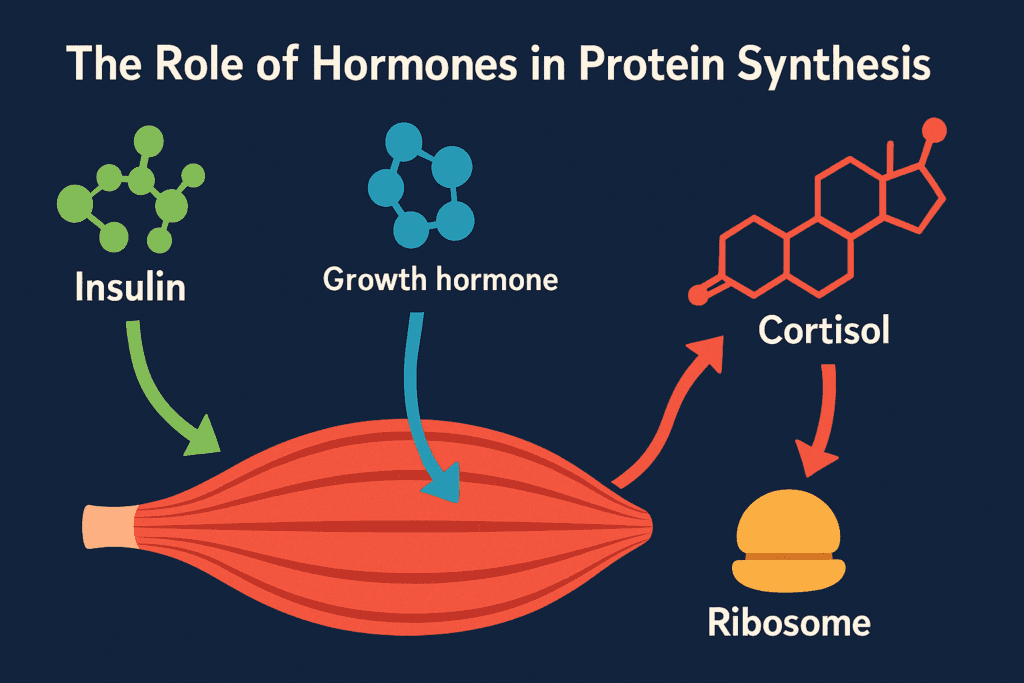
The Role of Hormones in Protein Synthesis
Protein synthesis is not solely a mechanical process; it is dynamically regulated by hormones that signal when and where proteins are made. Insulin, for example, promotes amino acid uptake and enhances mTOR signaling, thus facilitating muscle growth and tissue repair. Growth hormone and testosterone also upregulate protein synthesis, making them particularly important in developmental stages and athletic training. On the other hand, stress hormones like cortisol can have catabolic effects, breaking down proteins to mobilize amino acids for immediate energy needs. These opposing hormonal influences reveal the finely tuned nature of protein synthesis regulation, and they highlight the importance of a balanced lifestyle that includes good nutrition, stress management, and physical activity to optimize hormonal profiles and support healthy protein production.
Cellular Quality Control and Protein Turnover
Once proteins are made, the body employs strict quality control mechanisms to ensure their proper function. Misfolded or damaged proteins are identified and targeted for degradation through structures like the proteasome or the lysosome. This process, known as protein turnover, allows the body to recycle amino acids and maintain cellular health. Autophagy—a process where the cell digests its own components—also plays a role in eliminating defective proteins and sustaining metabolic balance. An imbalance in protein synthesis and degradation can lead to accumulation of abnormal proteins, contributing to conditions like Alzheimer’s disease, Parkinson’s disease, and certain forms of cancer. Therefore, understanding not just where proteins are made but also how they are maintained and regulated adds a critical layer to the broader conversation about protein metabolism.
The Impact of Inflammation and Illness on Protein Production
Chronic inflammation and illness can severely disrupt the body’s ability to make proteins. During acute illness or infection, the body prioritizes immune protein production over structural or muscle protein synthesis. Inflammatory cytokines can suppress mTOR signaling and promote muscle breakdown, leading to a catabolic state. In cases of prolonged illness or autoimmune disease, persistent inflammation may continuously impair protein metabolism, resulting in muscle wasting, fatigue, and weakened immune function. Nutritional intervention, including high-protein diets and anti-inflammatory nutrients like omega-3 fatty acids, can help mitigate these effects and support recovery. Understanding where proteins are made and how inflammation affects this process is particularly important in clinical nutrition and integrative health strategies.
Why Protein Synthesis Matters for Prevention and Wellness
Protein synthesis is central to nearly every aspect of human health. It governs not only muscle development and repair but also the production of enzymes, neurotransmitters, and hormones that regulate metabolism, mood, and immunity. Inadequate protein intake or impaired synthesis can lead to a host of health issues, including sarcopenia, poor wound healing, immune dysfunction, and chronic fatigue. In contrast, supporting the body’s ability to make proteins through balanced nutrition, physical activity, and stress management enhances wellness, longevity, and resilience. In this context, knowing where proteins are made becomes more than a biochemical curiosity—it becomes a practical foundation for lifestyle interventions that promote health and prevent disease.
Connecting Diet to Molecular Function: Practical Takeaways
Eating a protein-rich diet is only one part of the equation; the quality, diversity, and timing of protein intake also influence how the body makes proteins. Complete proteins, found in animal products and some plant-based combinations, provide all essential amino acids necessary for optimal synthesis. Spacing protein intake evenly throughout the day—as opposed to consuming most protein in a single meal—has been shown to improve net protein balance. In addition, combining protein intake with resistance training enhances muscle protein synthesis more than either stimulus alone. These strategies help optimize the efficiency of protein production and highlight the synergy between diet and lifestyle factors in maintaining health. Ultimately, making informed nutritional choices can directly affect how, when, and where proteins are made within the body.
How Advances in Science Are Enhancing Our Understanding of Protein Synthesis
Recent scientific developments have greatly expanded our understanding of protein synthesis. Techniques such as ribosome profiling and CRISPR gene editing are allowing researchers to observe translation in real-time and manipulate genetic sequences to study protein function. Insights into the regulation of protein synthesis pathways are paving the way for therapeutic strategies targeting diseases of protein misfolding and metabolic dysfunction. In the realm of personalized nutrition, genetic testing and biomarker analysis are helping tailor protein recommendations based on individual metabolic needs and genetic predispositions. As we learn more about where proteins are made and the variables that influence their production, the potential for targeted, preventative health strategies becomes increasingly powerful.
The Future of Nutrition in Supporting Protein Synthesis
As nutritional science evolves, so does our capacity to influence protein synthesis through diet. Emerging trends in medical nutrition therapy emphasize the importance of personalized dietary interventions based on genomic, metabolomic, and microbiome data. These tools help identify nutrient deficiencies, amino acid imbalances, and metabolic inefficiencies that may impair the body’s ability to make proteins. Innovations in food technology, such as plant-based meat analogs and protein-fortified products, offer new ways to meet protein requirements across diverse populations. Additionally, novel supplements like bioactive peptides and branched-chain amino acid formulas are being explored for their potential to stimulate muscle synthesis and enhance recovery. These advances are rooted in a deeper understanding of where proteins are made and how that knowledge can be applied to improve health outcomes.
Frequently Asked Questions: How Your Body Makes Proteins and Where Proteins Are Made
1. Can stress or lack of sleep affect how the body makes proteins? Absolutely. Chronic stress and poor sleep hygiene have been shown to disrupt hormonal balance, particularly through increased cortisol levels, which can impair the body’s ability to make proteins efficiently. Sleep deprivation reduces the release of growth hormone, which plays a pivotal role in tissue repair and protein synthesis. When cortisol levels rise and anabolic hormones fall, the body may prioritize energy conservation over the production of new proteins. Over time, this imbalance can compromise muscle maintenance and immune function. It’s essential to understand that optimal protein synthesis requires not only sufficient nutrition but also healthy sleep patterns and stress management, which influence the conditions under which the body makes proteins.
2. How do intermittent fasting and time-restricted eating impact where proteins are made? Emerging evidence suggests that intermittent fasting can enhance the efficiency of protein synthesis by optimizing insulin sensitivity and activating autophagy pathways. When fasting is followed by nutrient-rich meals, particularly those high in protein, it can create a favorable environment for the ribosomes—where proteins are made—to operate with increased precision. However, prolonged fasting without adequate refeeding may inhibit anabolic processes, especially in older adults or individuals with high physical demands. Strategic timing of protein intake around fasting windows is key to ensuring that the body makes proteins effectively during feeding periods. These patterns underscore the intricate relationship between metabolic cycles and the cellular sites where proteins are made.
3. Are there diseases or genetic conditions that alter where proteins are made or how they are synthesized? Yes, several genetic and acquired conditions interfere with the cellular machinery responsible for protein synthesis. For instance, ribosomopathies are rare disorders in which mutations disrupt the function of ribosomes—the structures where proteins are made—leading to abnormalities in growth, immunity, and even cancer predisposition. Likewise, mitochondrial diseases can affect energy availability for protein synthesis, particularly in tissues with high energy demands. Even conditions like cystic fibrosis and some cancers involve errors in protein folding or misdirection after proteins are made, resulting in cellular dysfunction. Understanding these pathologies offers new insight into the fragility and complexity of the processes that make proteins.
4. What role does gut health play in how the body makes proteins? Gut health is increasingly recognized as a foundational factor in nutrient absorption, inflammation regulation, and immune signaling—all of which influence protein metabolism. A healthy gut ensures optimal breakdown and absorption of amino acids, the fundamental materials needed to make proteins. In contrast, gastrointestinal disorders like Crohn’s disease, celiac disease, or chronic dysbiosis may compromise nutrient uptake, thereby limiting the substrate available for synthesis. Furthermore, gut microbes themselves may secrete compounds that impact the efficiency of ribosomes, where proteins are made, particularly through modulation of host gene expression. Supporting the gut microbiome with fiber-rich foods, probiotics, and anti-inflammatory nutrients can indirectly enhance how effectively the body makes proteins.
5. How does aging affect the structures where proteins are made? As the body ages, several changes occur at the cellular level that reduce the efficiency of protein synthesis. Ribosomes—the organelles where proteins are made—may become less efficient due to oxidative damage, altered signaling pathways, or decreased ribosomal biogenesis. Additionally, anabolic resistance in older adults means that even when adequate protein is consumed, the body may not make proteins as readily as it did in youth. These effects can contribute to age-related muscle loss, known as sarcopenia. Counteracting this decline involves resistance training, sufficient leucine intake, and maintaining metabolic health to preserve the function of the cellular structures that make proteins.
6. Can plant-based diets support efficient protein synthesis even though plant proteins are sometimes considered incomplete? Yes, well-planned plant-based diets can fully support the body’s ability to make proteins. While some plant proteins may lack one or more essential amino acids, combining complementary sources—such as legumes with grains—can provide all the necessary building blocks. Additionally, the presence of phytonutrients and fiber in plant-based diets can support digestive and metabolic processes that enhance absorption and cellular efficiency. Ensuring adequate protein quantity and diversity is essential, especially for supporting the cellular sites where proteins are made, such as the ribosomes and rough endoplasmic reticulum. With careful planning, a plant-based diet can be just as effective in supporting the body’s efforts to make proteins.
7. Are there medical tests that can evaluate how well your body makes proteins? While there is no single test that captures the entirety of protein synthesis, several clinical markers can offer indirect insights. Serum albumin and prealbumin levels can reflect the body’s protein status, especially during illness or recovery. More specialized tests like nitrogen balance assessments or isotopic tracer studies can evaluate how efficiently the body makes proteins under controlled conditions. Emerging technologies, such as ribosome profiling, provide detailed snapshots of active translation and where proteins are made at the molecular level, though these are primarily used in research. These tools are valuable in clinical and academic settings to assess whether nutritional interventions are effectively supporting protein synthesis.
8. How does physical exercise influence where proteins are made in the body? Exercise, especially resistance training, stimulates protein synthesis by increasing the activation of mTOR pathways and promoting the transcription of genes associated with muscle repair. After physical activity, the demand for new proteins rises, prompting increased activity in ribosomes, where proteins are made, particularly in muscle cells. This adaptive response enhances muscle strength, recovery, and endurance over time. Exercise also improves cellular signaling and insulin sensitivity, both of which create a favorable environment for the body to make proteins efficiently. For athletes or those in rehabilitation, optimizing the timing and composition of post-exercise meals can further support the cellular machinery that makes proteins.
9. Do protein supplements target the places where proteins are made, or are they just general fuel? Protein supplements act as a readily digestible source of amino acids that support the body’s broader metabolic needs, but their utility in targeting where proteins are made lies in how and when they are used. Fast-digesting proteins like whey can elevate blood amino acid levels quickly, directly stimulating the mTOR pathway and activating ribosomes—where proteins are made—especially after resistance training. Some advanced formulations now include bioactive peptides or amino acid blends designed to enhance uptake by muscle tissue or stimulate hormone release. However, supplements alone are not a magic bullet; they are most effective when combined with strategic nutrition, physical activity, and adequate recovery to ensure that the body makes proteins where and when they are needed most.
10. What innovations in biotechnology are changing our understanding of where proteins are made and how they function? Biotechnological advances such as CRISPR gene editing, single-cell RNA sequencing, and ribosome footprinting are offering unprecedented insights into protein synthesis. Scientists can now observe in real-time which genes are being translated and how different conditions influence where proteins are made within specific cell types. Synthetic biology is even exploring how to reengineer ribosomes or create new types of translational machinery that could outperform natural ones. This research has implications for treating genetic diseases, developing precision medicine, and understanding how the body makes proteins in complex and changing environments. The field is rapidly evolving, revealing that the traditional model of protein synthesis is far more dynamic and adaptable than previously thought.
Conclusion: Why Understanding Where Proteins Are Made Matters for Lifelong Health
At its core, the process of making proteins is a biological marvel that underpins virtually every function of the human body. From the nucleus to the ribosome, and through the endoplasmic reticulum and Golgi apparatus, the precision with which the body makes proteins is nothing short of extraordinary. Yet this intricate process is deeply influenced by the choices we make every day—what we eat, how we move, how we sleep, and how we manage stress. Knowing where proteins are made is not just an academic detail; it is an essential insight that empowers individuals to take control of their health. By supporting the body’s protein synthesis pathways through high-quality nutrition, balanced lifestyle practices, and informed health decisions, we can foster resilience, enhance recovery, and optimize performance at every stage of life. Whether you are an athlete, a healthcare provider, or someone simply striving to feel better, the understanding of how the body makes proteins—and where proteins are made—is a critical key to unlocking lifelong wellness.
Further Reading:
9 Important Functions of Protein in Your Body
How much protein do you need every day?