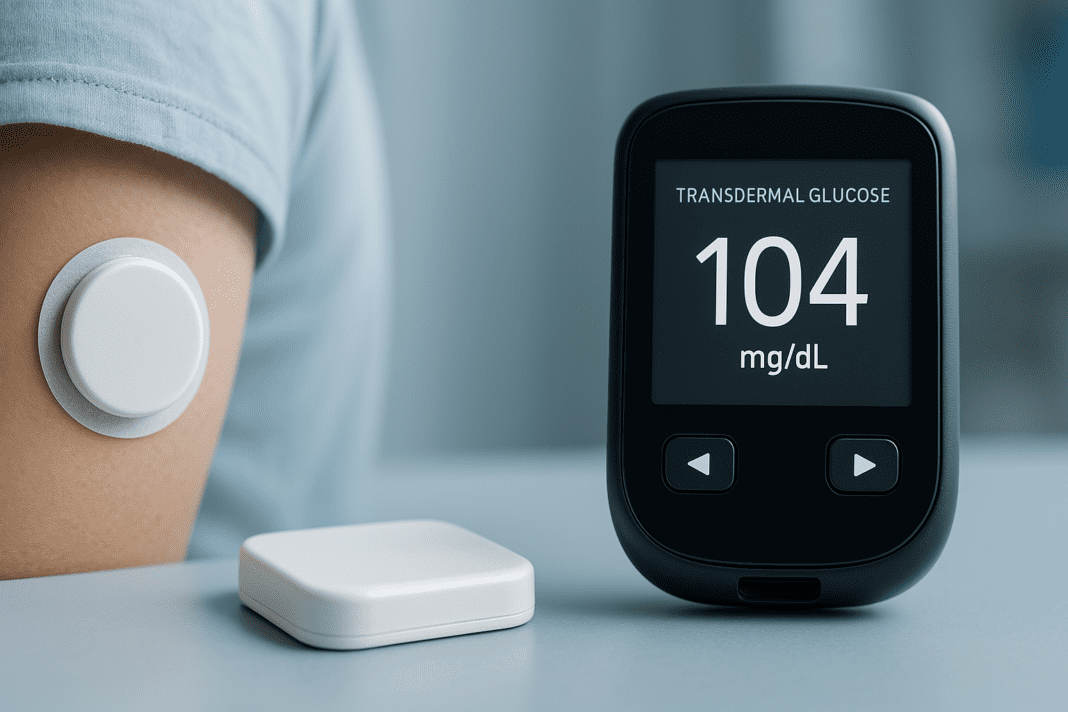
The Promise of Innovation in Diabetes Management
Diabetes, a chronic metabolic disorder characterized by abnormal glucose regulation, affects hundreds of millions worldwide and remains one of the most burdensome conditions in modern healthcare. With the global prevalence of both type 1 and type 2 diabetes steadily rising, the urgency for innovative, noninvasive, and more accessible monitoring methods has never been greater. Traditional glucose monitoring, while effective, often demands finger pricks multiple times a day, leading to discomfort, noncompliance, and decreased quality of life for many patients. Against this backdrop, the emergence of transdermal glucose sensor technology offers a revolutionary shift in how blood sugar can be tracked and managed.
You may also like: Breakthroughs in Current Diabetes Research: What the Latest Studies Reveal About Treatment and Prevention
The medical community has long sought to improve upon the classic glucose meter model. While many people are familiar with the basic glucose monitor name brands such as Accu-Chek or OneTouch, these devices are largely dependent on capillary blood samples. This invasive requirement underscores the need for a paradigm shift. As researchers push the boundaries of noninvasive monitoring technologies, transdermal glucose sensors—devices that measure glucose levels through the skin without breaking it—are rising to the forefront of biomedical innovation.
Understanding the Evolution of Blood Sugar Monitoring
To fully appreciate the impact of current innovations, it is essential to understand the historical trajectory of glucose monitoring. Initially, urine testing was the standard for diabetes management. While crude and largely inaccurate, it provided a rudimentary method for detecting glucose. This was eventually replaced by capillary blood testing using glucometers, which allowed patients to obtain real-time blood glucose readings using disposable test strips. These devices revolutionized self-management and formed the foundation of modern diabetes care.
Despite their utility, the limitations of conventional blood glucose meters are widely recognized. Not only do they require frequent finger pricks, but they also provide single-point-in-time data, which may not accurately reflect glucose variability throughout the day. To address this gap, continuous glucose monitoring (CGM) systems were developed, offering a more dynamic and comprehensive view of glucose trends. Devices like the Freestyle Libre and Dexcom G6 exemplify the evolution toward semi-invasive, real-time tracking, using a subcutaneous blood sugar monitor that measures interstitial fluid glucose levels.
However, even CGMs come with drawbacks. The need for skin puncture, risk of infection, and limited sensor wear time present practical and psychological barriers for some patients. This is where transdermal glucose sensors begin to show their promise, offering noninvasive yet accurate readings without the burdensome trade-offs of existing systems.

What Makes Transdermal Glucose Sensors Different?
At their core, transdermal glucose sensors represent a leap forward in the attempt to monitor glucose levels painlessly and continuously. These sensors work by detecting glucose molecules that diffuse through the skin using a variety of techniques—such as reverse iontophoresis, spectroscopy, and fluorescence-based technologies. What differentiates them from a subcutaneous blood sugar monitor is that they do not penetrate the skin, eliminating the need for needles or implantable devices.
This noninvasive nature reduces the risk of infection and minimizes discomfort, making glucose monitoring more accessible and appealing for both patients and providers. Furthermore, the design of these devices typically integrates with wearable technology, allowing users to track their data in real time through smartphone applications or smartwatches. This seamless integration is more than a convenience—it supports more consistent monitoring, improves patient engagement, and contributes to better glycemic control overall.
The refinement of the transdermal blood glucose monitor is also heavily reliant on advancements in material science and microelectronics. Flexible biosensors made from graphene or nanomaterials are enabling the creation of ultra-thin, breathable patches that can detect minute changes in glucose concentrations with impressive precision. These patches are also designed to remain functional for extended periods, enhancing user convenience and reducing device costs over time.
Key Players and Promising Technologies in Development
Several academic and industrial research teams are racing to perfect the next generation of noninvasive glucose monitoring technologies. Companies like GlucoTrack, Nemaura Medical, and SugarBEAT have made significant strides with transdermal glucose sensor prototypes that are currently in clinical trials or early commercial release phases. These devices use a range of techniques, such as ultrasound and impedance spectroscopy, to estimate blood glucose levels from outside the skin.
Researchers are also exploring wearable patches embedded with microfluidic systems and optical sensors to monitor glucose noninvasively. These patches represent an exciting frontier in biotechnology. Some are even powered by body heat or kinetic energy, reducing the need for frequent recharging and making them more viable for long-term, daily use. At the academic level, institutions like MIT, Stanford, and the University of Tokyo have published groundbreaking studies on the application of nanomaterials in creating high-fidelity transdermal glucose sensors.
A particularly exciting development is the diabetes chip—miniaturized biosensors that can be integrated into transdermal patches or contact lenses to provide real-time glucose monitoring without user input. These diabetes chips could mark a transformative milestone by offering continuous data collection that aligns seamlessly with AI-powered decision support systems.
FDA Oversight and the Push for Standardization
While the innovation race accelerates, regulatory oversight remains crucial to ensure safety, accuracy, and reliability. The U.S. Food and Drug Administration (FDA) plays a central role in evaluating these new technologies. Devices must meet stringent performance benchmarks before being included in the list of FDA approved glucose meters, especially when marketed as medical-grade tools for diabetes management.
The FDA approval process ensures that patients are protected from untested or underperforming products that could jeopardize health outcomes. Inclusion in the list of FDA approved glucose meters is not just a technical certification; it represents a critical step in building trust among clinicians, payers, and patients. It also enables insurance coverage and broader clinical adoption, which are vital for mainstreaming any new technology in the healthcare system.
Manufacturers aiming to produce a reliable transdermal blood glucose monitor must therefore navigate complex regulatory pathways, often requiring extensive clinical validation and post-market surveillance. In doing so, these companies not only demonstrate their commitment to safety and efficacy but also contribute to a more transparent and robust healthcare ecosystem.
Overcoming Technical and Clinical Challenges
Despite the promise of transdermal sensors, several technical and clinical hurdles remain. Skin, as a biological barrier, can introduce variability in glucose diffusion rates due to factors like sweat, hydration levels, and skin thickness. This makes calibration a significant challenge, as sensor readings must accurately reflect blood glucose levels despite external interferences.
Additionally, the signal strength generated by noninvasive sensors can be weaker than that from traditional subcutaneous blood sugar monitors. Researchers are addressing this by developing more sensitive detection algorithms and noise-filtering techniques, but the complexity of human skin physiology continues to pose obstacles. Consistency of readings across different body types, ages, and environmental conditions is another area under active investigation.
Moreover, ensuring the long-term durability of these devices without compromising their sensitivity is crucial. Frequent device malfunction or degradation could deter users from adopting the technology. To address this, new biosensors are being engineered to be both biocompatible and mechanically robust, supporting longer wear times without sacrificing data accuracy.

Patient-Centered Design and Accessibility Considerations
Beyond the laboratory and clinical trial settings, the true success of any new diabetes monitoring device depends on how well it meets the needs of real-world users. Patient-centered design is increasingly recognized as a fundamental principle in medical technology development. Factors such as comfort, ease of use, affordability, and aesthetics can greatly influence whether patients adhere to a new monitoring regimen.
One of the most powerful benefits of a wearable transdermal glucose sensor is its ability to integrate discreetly into everyday life. Whether embedded into a skin patch or worn as a smartwatch accessory, these devices avoid the stigma and inconvenience often associated with traditional glucose testing methods. In turn, this may encourage more frequent glucose checks, improving glycemic control and reducing long-term complications.
Affordability remains a pressing concern, particularly for underserved populations who may not have access to the latest medical innovations. Ensuring that transdermal glucose sensors are not only technologically advanced but also economically accessible will be essential in democratizing diabetes care. Healthcare systems and insurance providers will need to evaluate cost-effectiveness models to justify coverage and reimbursement.
The Role of Data Analytics and AI Integration
As transdermal glucose sensors become more widespread, the volume of real-time data generated by these devices will grow exponentially. This data holds immense potential to enhance personalized diabetes management through predictive analytics and artificial intelligence (AI). Machine learning algorithms can detect patterns in glucose fluctuations, predict future trends, and provide tailored recommendations for insulin dosing, meal planning, and activity adjustments.
The integration of AI with wearable technology also opens new frontiers in preventive care. By analyzing data from a transdermal blood glucose monitor in conjunction with other health metrics—such as heart rate, sleep quality, and physical activity—clinicians can gain a more comprehensive view of patient health. This holistic perspective enables proactive interventions before problems escalate, potentially reducing hospitalizations and improving quality of life.
Moreover, cloud-based platforms that aggregate and analyze glucose data can facilitate remote monitoring by healthcare providers. This is particularly valuable for patients in rural or underserved areas who may not have regular access to endocrinologists. Real-time alerts, automated reports, and virtual consultations could all become standard components of diabetes care in the near future.
Bridging the Gap Between Innovation and Implementation
Translating technological breakthroughs into everyday clinical practice requires more than scientific excellence. Healthcare providers must be trained to understand and utilize these new tools effectively. Education initiatives aimed at clinicians and patients alike will play a critical role in building confidence in the accuracy and utility of transdermal glucose sensors.
Public health campaigns and clinical guidelines will also need to evolve to accommodate these advances. Including transdermal technologies in standard treatment algorithms and care pathways could hasten their adoption and improve outcomes across diverse patient populations. In this context, collaboration among researchers, industry leaders, regulatory agencies, and patient advocacy groups becomes paramount.
The broader vision extends beyond simply replacing finger pricks. It encompasses a new model of chronic disease management—one that is continuous, connected, and customized to the individual. With each refinement, each successful trial, and each regulatory milestone, the path toward mainstream adoption becomes clearer.

Frequently Asked Questions (FAQ): Emerging Trends in Noninvasive Glucose Monitoring
What makes a transdermal glucose sensor ideal for pediatric diabetes patients?
Children with diabetes often face unique challenges when it comes to traditional blood sugar monitoring. Many are reluctant to use finger-prick tests due to discomfort, and subcutaneous devices can be intimidating or even painful. A transdermal glucose sensor, being completely noninvasive, offers a pain-free alternative that improves adherence in younger patients. These sensors can be integrated into fun, child-friendly wearables—like stickers or wristbands—making the monitoring process feel less clinical and more routine. Importantly, integrating a transdermal blood glucose monitor with parental apps allows caregivers to track readings remotely, reducing the need for constant supervision while ensuring safety.
How are diabetes chips being used in next-generation smartwatches?
Diabetes chip technology is becoming central to the evolution of smartwatches aimed at health-conscious users, especially those managing chronic conditions. These micro-scale biosensors can now be embedded directly into the watch face or band, allowing users to continuously monitor glucose without additional patches or implants. When paired with a transdermal glucose sensor, the diabetes chip enables accurate, real-time data capture that syncs directly with mobile health platforms. In advanced models, algorithms interpret data trends and offer personalized recommendations. This not only enhances user convenience but also builds a more intuitive and proactive approach to managing diabetes.
Are there any risks associated with relying on a transdermal blood glucose monitor for insulin dosing?
Although transdermal blood glucose monitors show significant promise, relying solely on them for insulin dosing should be approached cautiously. While they eliminate the discomfort of traditional methods, calibration issues or delayed response to rapid glucose fluctuations may affect their real-time accuracy. Experts recommend using them alongside a validated glucose monitor name on the list of FDA approved glucose meters until sufficient individual reliability has been established. Furthermore, manufacturers continue to refine sensor sensitivity to bridge this gap. As with all medical devices, ongoing consultation with a healthcare provider is essential when using a new monitoring method for therapeutic decision-making.
What should consumers consider when comparing glucose monitor name brands?
When exploring options across various glucose monitor name brands, consumers should prioritize accuracy, ease of use, and compatibility with their lifestyle. Some patients may prefer compact, travel-friendly designs, while others may seek integration with wearable technology. It’s also important to verify whether the device appears on the list of FDA approved glucose meters, which ensures clinical validation. Transdermal glucose sensor models may not always be included yet, depending on their regulatory status, but newer hybrid systems are emerging that combine subcutaneous blood sugar monitor technology with transdermal innovations for added reliability. Long-term cost, data-sharing features, and customer support are additional factors to assess.
Can athletes benefit from using a subcutaneous blood sugar monitor or transdermal sensor?
Yes, athletes managing diabetes often benefit significantly from wearable glucose technology, including both subcutaneous blood sugar monitors and newer transdermal glucose sensors. Physical exertion can lead to unpredictable glucose fluctuations, and continuous monitoring allows for timely adjustments to avoid performance dips or medical emergencies. A subcutaneous blood sugar monitor provides highly responsive data, while a transdermal option offers comfort during prolonged activities where skin irritation or friction is a concern. Emerging transdermal systems are now designed with waterproofing and flexible adhesives to accommodate high-movement scenarios. Coupled with smartphone alerts, these tools empower athletes to train and compete with greater confidence and fewer interruptions.
How are transdermal glucose sensors addressing disparities in diabetes care access?
Transdermal glucose sensors are playing a growing role in improving health equity, especially in underserved or rural communities. Traditional monitoring systems often come with recurring costs for test strips or insertion sets, which can be financially burdensome. A well-designed transdermal blood glucose monitor minimizes these consumables, making long-term management more affordable. Moreover, these devices are typically user-friendly, requiring minimal training—an important consideration in low-resource settings. Partnerships between device manufacturers and public health programs are also emerging to subsidize access, particularly where diabetes prevalence intersects with economic hardship.
What role does data security play in the use of wearable diabetes technology?
With the rise of connected devices such as those using a diabetes chip or transdermal glucose sensor, data security has become a pressing issue. These devices collect sensitive health metrics continuously, which must be protected to ensure user privacy and compliance with health data regulations like HIPAA. Manufacturers are now integrating end-to-end encryption and multi-factor authentication into device platforms. Cloud-based dashboards that pull data from a subcutaneous blood sugar monitor or a transdermal blood glucose monitor are also incorporating anonymized aggregation tools for research purposes. Consumers should be proactive in understanding how their data is stored, who can access it, and what options exist for revoking sharing permissions.
How might AI reshape the clinical interpretation of transdermal glucose data?
Artificial intelligence is redefining how clinicians interpret glucose data from noninvasive devices like transdermal glucose sensors. Instead of viewing static readings, clinicians can now leverage AI-driven models that analyze data trends from transdermal blood glucose monitors and predict glycemic excursions before they happen. When combined with information from a diabetes chip embedded in wearables, these insights become even more granular. This predictive capacity helps clinicians tailor treatment strategies more precisely, reducing the burden of trial-and-error in medication adjustments. As algorithms mature, expect more personalized alerts, dosage optimization, and automated care suggestions based on continuously updated patient profiles.
Why aren’t all transdermal glucose sensor systems yet on the list of FDA approved glucose meters?
While the transdermal glucose sensor market is expanding rapidly, not all systems are immediately listed among the list of FDA approved glucose meters due to the rigorous validation required. FDA approval involves extensive clinical trials, performance benchmarks, and long-term safety assessments, especially for devices influencing insulin therapy decisions. Many promising transdermal blood glucose monitor prototypes are still undergoing these evaluations. Additionally, some devices prioritize consumer wellness tracking over medical-grade precision, which can limit their eligibility for inclusion. That said, companies working toward FDA approval are continually publishing peer-reviewed results to demonstrate efficacy, which accelerates the regulatory pathway.
How do diabetes chips enhance the accuracy of glucose monitoring systems?
Diabetes chips serve as highly sensitive biosensors capable of detecting minuscule biochemical changes in interstitial or dermal fluid. When embedded into a subcutaneous blood sugar monitor or transdermal glucose sensor, they improve the fidelity of glucose readings by filtering out noise and correcting for environmental interference. Some advanced chips include onboard processors that pre-analyze data before it’s transmitted to an external app, increasing reliability even in unstable conditions. In multi-sensor platforms, a diabetes chip may work alongside other physiological monitors—like heart rate or hydration sensors—to provide contextual data for smarter interpretations. These innovations are helping bridge the gap between noninvasive convenience and clinical-grade accuracy.
The Future of Noninvasive Glucose Monitoring: A New Era of Personalized Diabetes Care
The evolution from traditional finger-prick glucometers to continuous glucose monitors has already marked a significant leap forward in diabetes care. But the rise of transdermal glucose sensors signals an even more transformative moment—one in which pain-free, noninvasive, real-time monitoring could become the norm rather than the exception. As researchers and developers work to refine this technology, its potential to enhance patient comfort, increase adherence, and reduce complications is coming into sharper focus.
This paradigm shift is not just about convenience; it’s about enabling a future in which blood glucose management is seamlessly woven into daily life. With the aid of wearable biosensors, advanced diabetes chips, and AI-enhanced analytics, patients could soon have unprecedented control over their condition. For clinicians, these tools offer a window into patient behaviors and physiology that can guide more personalized, effective interventions.
As more products receive regulatory approval and are added to the growing list of FDA approved glucose meters, trust in these innovations will deepen. At the same time, expanding insurance coverage, refining clinical guidelines, and ensuring equitable access will be vital in delivering on the promise of these technologies. The journey from the earliest glucose monitor name brands to today’s sophisticated, skin-friendly devices reflects not only scientific progress but also a deeper understanding of what it means to live with diabetes.
The future is undeniably bright for noninvasive glucose monitoring. As transdermal glucose sensor technology continues to mature, it holds the power to reshape diabetes care on a global scale—making it smarter, more humane, and better aligned with the needs of the people it serves.
noninvasive glucose tracking, wearable diabetes devices, smart health sensors, real-time blood sugar data, continuous glucose analytics, painless glucose testing, diabetic wearable technology, glucose monitoring advancements, skin sensor for glucose, AI in diabetes care, digital health monitoring, glucose prediction algorithms, health tech for diabetics, remote patient glucose tracking, biosensor technology in diabetes, nanotech diabetes sensors, mobile apps for glucose tracking, personalized diabetes management, innovation in diabetic care, diabetes wearable innovation
Further Reading:
Blood glucose sensors and recent advances: A review
Wearable non-invasive glucose sensors based on metallic nanomaterials
Minimally and non-invasive glucose monitoring: the road toward commercialization
Disclaimer
The information contained in this article is provided for general informational purposes only and is not intended to serve as medical, legal, or professional advice. While MedNewsPedia strives to present accurate, up-to-date, and reliable content, no warranty or guarantee, expressed or implied, is made regarding the completeness, accuracy, or adequacy of the information provided. Readers are strongly advised to seek the guidance of a qualified healthcare provider or other relevant professionals before acting on any information contained in this article. MedNewsPedia, its authors, editors, and contributors expressly disclaim any liability for any damages, losses, or consequences arising directly or indirectly from the use, interpretation, or reliance on any information presented herein. The views and opinions expressed in this article are those of the author(s) and do not necessarily reflect the official policies or positions of MedNewsPedia.