Introduction: The Convergence of Two Global Epidemics
Diabetes and cancer are among the most pressing global health concerns of the 21st century. Both diseases impose significant burdens on patients, families, and healthcare systems alike. While each condition has traditionally been treated as a distinct entity, recent scientific inquiry has begun to uncover a more intimate connection between them. Specifically, a growing body of research suggests that metabolic disorders, such as type 2 diabetes, may increase the risk of developing certain forms of cancer. This raises an important and medically complex question: can diabetes lead to cancer? Exploring this issue requires a closer look at the molecular, epidemiological, and clinical data that illuminate the relationship between chronic hyperglycemia and tumor biology.
You may also like: Breakthroughs in Current Diabetes Research: What the Latest Studies Reveal About Treatment and Prevention
Understanding the intricacies of this connection is essential for clinicians, researchers, and patients striving to manage long-term health outcomes. From insulin resistance to inflammation and beyond, the physiological hallmarks of diabetes intersect with key mechanisms known to drive carcinogenesis. As evidence continues to accumulate, it becomes increasingly clear that addressing diabetes may not only improve glycemic control but also contribute to cancer prevention strategies. In this article, we dive into the current landscape of medical research to examine whether diabetes can increase cancer risk, with special attention to emerging studies, plausible biological mechanisms, and implications for public health.
Understanding Diabetes and Its Systemic Effects
To fully appreciate the link between diabetes and cancer, one must first understand the systemic nature of diabetes itself. Diabetes mellitus is not simply a disease of high blood sugar; it is a complex metabolic disorder that affects virtually every organ system. In type 1 diabetes, the immune system destroys insulin-producing beta cells in the pancreas, leading to absolute insulin deficiency. In contrast, type 2 diabetes is characterized by insulin resistance and relative insulin insufficiency, often in the context of obesity and chronic low-grade inflammation.
The long-term complications of diabetes extend beyond glucose regulation. Persistent hyperglycemia damages blood vessels, nerves, and organs through a process known as glycation, which alters proteins and disrupts cellular function. Additionally, insulin resistance is often accompanied by hyperinsulinemia—chronically elevated levels of insulin in the bloodstream. Insulin, a growth-promoting hormone, can have mitogenic effects that may influence cancer development. Thus, the biological disturbances of diabetes—chronic inflammation, oxidative stress, insulin signaling dysregulation, and adipose tissue dysfunction—can create an internal environment conducive to tumor growth.
Can Diabetes Lead to Cancer? An Epidemiological Perspective
When asking whether diabetes can lead to cancer, population-level studies offer compelling, though nuanced, answers. Large-scale epidemiological analyses consistently show that individuals with diabetes face an increased risk of developing several types of cancer. These include cancers of the liver, pancreas, endometrium, colon, breast, and bladder. For example, a landmark meta-analysis published in Diabetologia reviewed data from over 100 studies and found that people with type 2 diabetes had a significantly higher incidence of liver and pancreatic cancers—raising urgent questions about shared pathophysiological pathways.
The diabetes-cancer connection is not uniformly distributed across all cancer types, however. Some cancers, such as prostate cancer, may exhibit lower incidence among diabetic patients, possibly due to hormonal changes or detection biases. Still, the prevailing trend underscores a statistically significant link between diabetes and increased cancer risk. Importantly, these findings are not merely artifacts of coexisting risk factors like obesity or sedentary lifestyle, though such variables do contribute to disease risk. Adjusted analyses that control for body mass index, age, smoking status, and physical activity still reveal elevated cancer risks in diabetic populations.
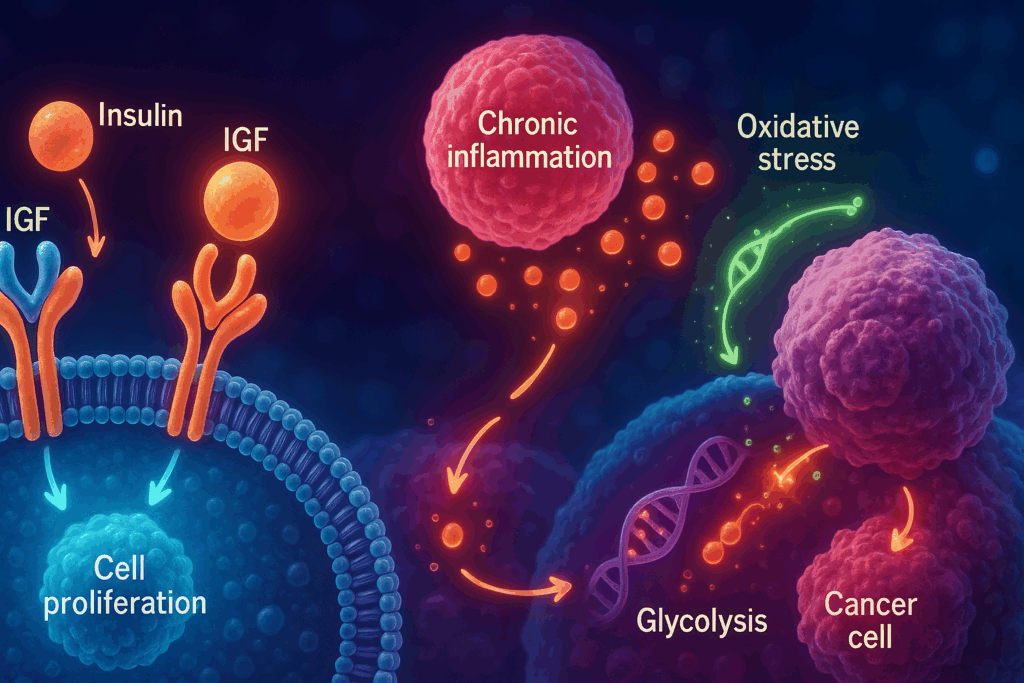
Exploring the Biological Mechanisms That Link Diabetes and Cancer
The question of whether diabetes causes cancer cannot be fully answered through epidemiology alone. To move beyond correlation and toward understanding causation, researchers have investigated the biological mechanisms that may mediate this relationship. One of the most prominent pathways involves insulin and insulin-like growth factors (IGFs). In individuals with type 2 diabetes, insulin resistance often leads to increased circulating levels of insulin, which can bind to insulin receptors and IGF receptors on cells, stimulating pathways that promote cell proliferation and inhibit apoptosis—key processes in carcinogenesis.
Another major contributor is chronic inflammation, a hallmark of both diabetes and cancer. High glucose levels can trigger pro-inflammatory cytokine release, creating a systemic environment that supports angiogenesis, cellular transformation, and tumor progression. Adipose tissue, especially in individuals with obesity-related diabetes, secretes inflammatory mediators such as TNF-alpha and interleukins, which have been implicated in cancer biology. Additionally, oxidative stress—a condition marked by excess reactive oxygen species—can damage DNA and contribute to genetic mutations that set the stage for cancer.
Furthermore, hyperglycemia itself may provide tumors with an abundant energy source. Cancer cells often rely on glycolysis—a phenomenon known as the Warburg effect—and persistently high blood glucose levels may enhance this metabolic reprogramming. As a result, diabetes may not only facilitate tumor initiation but also accelerate tumor progression through multiple, overlapping pathways.
Diabetes and Pancreatic Cancer: A Particularly Potent Link
Among the various malignancies associated with diabetes, pancreatic cancer stands out as particularly concerning. Pancreatic cancer is notoriously aggressive, with poor prognosis and limited treatment options. Studies have consistently reported that individuals with long-standing diabetes have an elevated risk of developing pancreatic cancer. More intriguingly, new-onset diabetes in older adults may sometimes be an early sign of undiagnosed pancreatic cancer, suggesting a bidirectional relationship.
In examining this association, researchers have uncovered several possible explanations. Chronic hyperglycemia and hyperinsulinemia may create a growth-favorable environment for neoplastic changes in pancreatic tissue. Additionally, inflammation and fibrosis in the pancreas—a common consequence of longstanding diabetes—can contribute to cellular dysfunction and malignant transformation. A study published in the Journal of the National Cancer Institute found that individuals with diabetes of more than five years’ duration had a significantly increased risk of developing pancreatic cancer, independent of other risk factors.
The relationship between diabetes and pancreatic cancer remains a major area of investigation. Researchers are actively exploring whether glycemic control, anti-diabetic medications, or earlier cancer screening could mitigate this risk. Although more work is needed, the connection between diabetes and pancreatic cancer underscores the importance of vigilant metabolic monitoring in at-risk populations.
Does Diabetes Lead to Cancer, or Are They Both Outcomes of a Common Pathology?
While many studies support the conclusion that diabetes can increase cancer risk, some researchers caution against oversimplifying the relationship. Instead of viewing diabetes as a direct cause of cancer, it may be more accurate to consider both conditions as outcomes of shared pathological mechanisms. Obesity, insulin resistance, dietary patterns, and sedentary behavior contribute to both diabetes and cancer risk, complicating attempts to establish direct causality.
The concept of “metabolic syndrome” offers a useful framework for understanding these overlapping risks. Metabolic syndrome refers to a constellation of abnormalities—including abdominal obesity, hypertension, dyslipidemia, and insulin resistance—that elevate the risk for both cardiovascular disease and certain cancers. In this context, diabetes and cancer are not sequential events but parallel consequences of an unhealthy metabolic environment. Nevertheless, even within this shared risk paradigm, the presence of diabetes independently increases the likelihood of developing specific cancers, suggesting that it is not merely a byproduct but an active player in carcinogenesis.
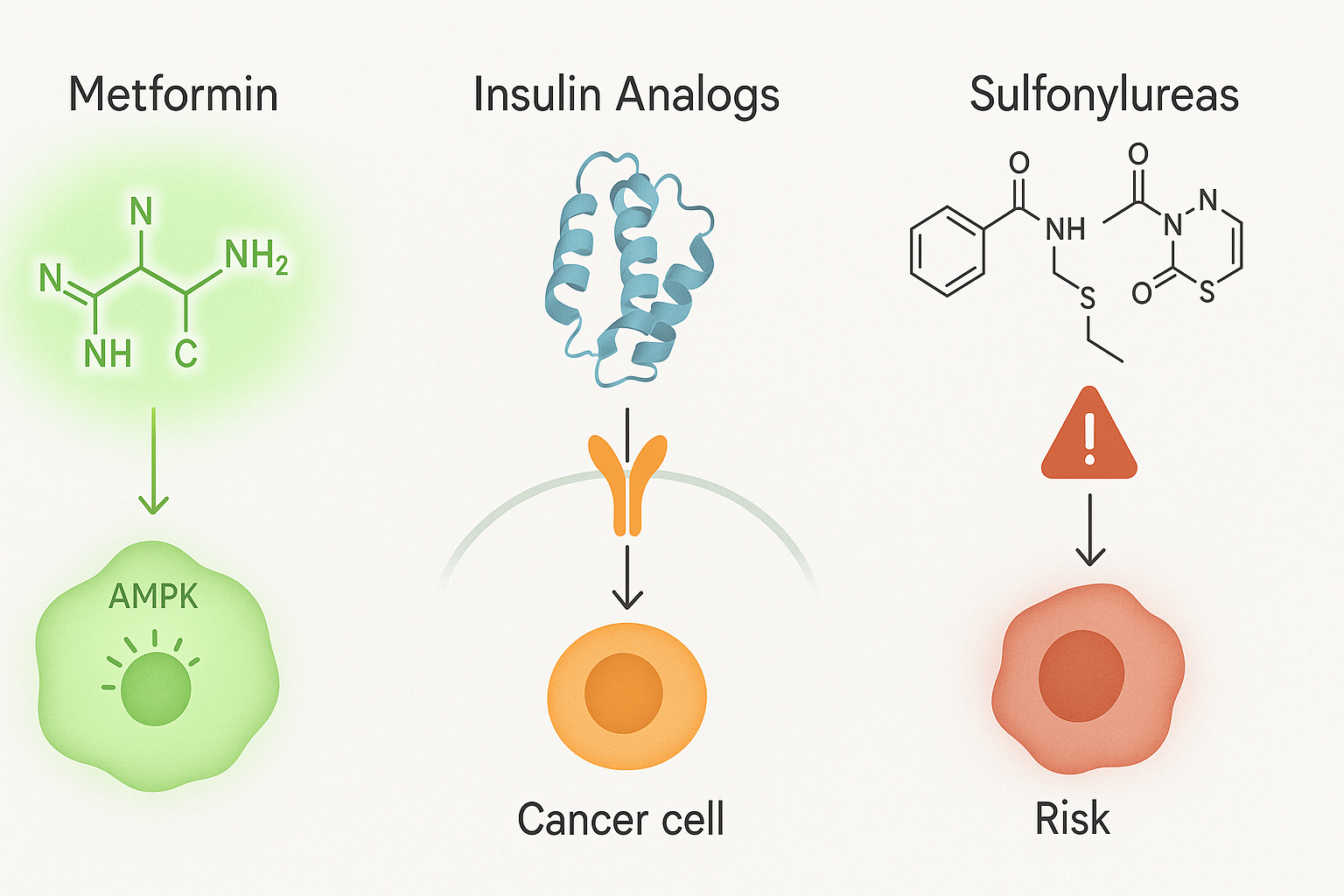
Anti-Diabetic Medications and Their Impact on Cancer Risk
The role of anti-diabetic medications in modifying cancer risk adds another layer of complexity to the discussion. Some drugs used to treat diabetes may influence cancer biology, either positively or negatively. For instance, metformin, a first-line treatment for type 2 diabetes, has attracted considerable interest for its potential anti-cancer properties. Metformin activates AMP-activated protein kinase (AMPK), a cellular energy sensor that inhibits the mTOR pathway—a key regulator of cell growth and proliferation. Observational studies have suggested that metformin use may be associated with reduced cancer incidence and improved survival in diabetic patients with cancer.
Conversely, other medications, such as insulin analogs and sulfonylureas, may have neutral or even adverse effects on cancer risk. Because these drugs increase circulating insulin levels, they may inadvertently promote tumorigenesis in susceptible individuals. While clinical data on this issue are mixed, the potential for drug-induced modulation of cancer risk highlights the need for individualized treatment approaches that consider long-term oncologic outcomes in addition to glycemic control.
Clinical Implications and Strategies for Prevention
Given the potential link between diabetes and cancer, what steps can clinicians and patients take to mitigate risk? First and foremost, effective diabetes management remains a cornerstone. Maintaining tight glycemic control, reducing insulin resistance, and preventing complications through lifestyle interventions and medications can all reduce the metabolic disturbances that may contribute to cancer development. Weight loss, regular physical activity, and a diet rich in whole grains, fruits, vegetables, and lean proteins are not only essential for managing diabetes but also offer protective effects against cancer.
For individuals with diabetes, cancer screening guidelines may warrant reevaluation. Early detection of cancers known to be associated with diabetes—such as colorectal, liver, and pancreatic cancer—could improve outcomes in this at-risk population. Additionally, clinicians should remain alert to signs of new-onset diabetes in older adults, particularly when accompanied by unexplained weight loss or gastrointestinal symptoms, as these may indicate underlying malignancy.
From a public health perspective, integrated strategies that address both diabetes and cancer prevention are urgently needed. This includes policies that promote healthier food environments, increase access to preventive care, and reduce disparities in chronic disease management. By recognizing the interconnection between these conditions, healthcare systems can develop more comprehensive models of care that address the root causes of metabolic dysfunction and its downstream consequences.
Emerging Research and Future Directions
The relationship between diabetes and cancer remains a dynamic area of research, with many unanswered questions. Scientists continue to investigate the molecular pathways that link hyperglycemia and insulin resistance to cancer initiation and progression. Genetic and epigenetic studies are shedding light on individual susceptibility, while new imaging techniques and biomarkers may improve early detection of diabetes-associated malignancies.
Precision medicine approaches, which tailor treatment based on individual genetic, metabolic, and environmental profiles, hold promise for optimizing care for patients at the intersection of diabetes and cancer. Clinical trials are also exploring whether targeted interventions—such as metformin use in non-diabetic individuals at high cancer risk—might have a preventive effect. In parallel, researchers are examining the role of the gut microbiome, mitochondrial function, and immune system interactions in mediating the diabetes-cancer connection.
As evidence accumulates, it is clear that addressing the question “does diabetes lead to cancer” involves a nuanced understanding of biology, behavior, and systemic health. While no single study can provide a definitive answer, the collective weight of the literature points to a meaningful and actionable relationship that demands ongoing attention from the medical community.
Frequently Asked Questions: Exploring the Diabetes-Cancer Connection
1. Are there specific lifestyle patterns in people with diabetes that may increase cancer risk?
Yes, lifestyle patterns commonly observed in people with diabetes may play a significant role in increasing cancer risk. Sedentary behavior, poor dietary choices high in ultra-processed foods, and inadequate physical activity are known contributors to both metabolic dysfunction and tumorigenesis. Chronic stress, poor sleep hygiene, and inconsistent medication adherence can further aggravate systemic inflammation and hormonal imbalances, creating conditions in which cancer cells may thrive. While these lifestyle elements are modifiable, they often remain under-addressed in clinical care. In light of ongoing research into whether and how diabetes can lead to cancer, personalized behavioral interventions targeting these modifiable risk factors are emerging as essential tools for cancer prevention.
2. Can diabetes lead to cancer through genetic predisposition or shared pathways?
Emerging research suggests that genetic predisposition may play a dual role in the development of both diabetes and cancer. Polymorphisms in genes related to insulin signaling, inflammation, and glucose metabolism have been identified in individuals who exhibit increased susceptibility to both diseases. Inherited mutations in tumor suppressor genes or DNA repair pathways can also be exacerbated by metabolic stress induced by diabetes. While the question “can diabetes lead to cancer” often emphasizes environmental or behavioral factors, a growing number of studies highlight the significance of genetic overlap and shared molecular pathways in understanding this dual risk. Future precision medicine strategies may rely on genetic screening to predict concurrent risks of diabetes and cancer in high-risk populations.
3. What role does the microbiome play in the relationship between diabetes and cancer?
The human gut microbiome is gaining recognition as a key player in the link between diabetes and cancer. Dysbiosis, or microbial imbalance, is commonly observed in individuals with type 2 diabetes and has been shown to affect insulin sensitivity and systemic inflammation. Certain microbial metabolites, such as secondary bile acids and short-chain fatty acids, can influence both glucose metabolism and tumor progression. Some strains of gut bacteria may even promote DNA damage or alter the immune system’s ability to recognize abnormal cells. The evolving science of microbiome research is expanding our understanding of how diabetes can lead to cancer through indirect, microbially mediated mechanisms, opening the door to potential probiotic or dietary interventions aimed at reducing cancer risk.
4. Does diabetes lead to cancer progression more aggressively than in non-diabetic individuals?
In some cases, yes—diabetes has been associated not only with increased cancer incidence but also with more aggressive tumor progression and poorer outcomes. Elevated glucose levels can serve as an abundant energy source for fast-growing cancer cells, accelerating tumor growth. Moreover, insulin resistance and high insulin levels may create a hormonal environment that fuels proliferation and metastasis. Several observational studies have found that diabetic individuals with cancer tend to have shorter survival times and less favorable responses to treatment compared to their non-diabetic counterparts. These findings support continued research into whether diabetes leads to cancer-related mortality through mechanisms beyond tumor initiation, including treatment resistance and impaired immune surveillance.
5. How does diabetes and pancreatic cancer represent a uniquely challenging relationship?
The link between diabetes and pancreatic cancer is especially complex and bidirectional. In many instances, long-standing type 2 diabetes increases the risk of developing pancreatic cancer due to chronic metabolic and inflammatory stress on pancreatic tissue. Conversely, in some patients, pancreatic cancer may present first as new-onset diabetes, particularly in older adults, as the tumor impairs insulin production. This overlapping presentation complicates early diagnosis and often delays treatment. Given the lethality of pancreatic cancer, understanding this dual relationship is critical to developing screening tools that can differentiate between typical type 2 diabetes and diabetes caused by underlying malignancy. Efforts are underway to create biomarkers that can signal when diabetes and pancreatic cancer may be co-occurring in their early, more treatable stages.
6. Are there psychosocial factors that may explain why diabetes cancer risks are often overlooked?
Yes, psychosocial factors such as health literacy, stigma, and medical fatigue can significantly impact the visibility of the diabetes cancer connection. Many patients with diabetes focus so heavily on glycemic control and cardiovascular complications that cancer prevention may be deprioritized. Moreover, the emotional burden of chronic disease management can lead to healthcare disengagement, reducing opportunities for cancer screening and risk education. From a clinical perspective, oncologists and endocrinologists often work in separate silos, which may limit interdisciplinary risk assessment. As public awareness grows about how diabetes can lead to cancer, more integrated models of care that consider psychosocial and systemic barriers will be essential.
7. How might advancements in wearable tech and continuous glucose monitoring impact our understanding of diabetes cancer risk?
Advances in wearable technologies and continuous glucose monitoring (CGM) systems offer a new frontier in assessing the dynamic interplay between glucose fluctuations and cancer risk. Real-time data collection allows researchers to examine not just average glucose levels, but also variability and patterns over time, which may correlate more closely with pro-carcinogenic environments. For instance, frequent glucose spikes and crashes may be more damaging than chronically elevated levels alone. By correlating CGM data with longitudinal cancer outcomes, researchers may soon uncover more precise indicators of when and how diabetes leads to cancer. These insights could ultimately guide personalized recommendations for maintaining metabolic stability in order to reduce cancer risk.
8. What are the implications of diabetes cancer research on global public health policy?
The intersection of diabetes and cancer has profound implications for global public health policy, particularly in low- and middle-income countries experiencing rapid increases in both conditions. Integrating cancer screening protocols into diabetes management programs could lead to earlier detection and improved outcomes in high-risk populations. Additionally, global food policy reform—focused on reducing sugar consumption and improving access to nutritious foods—may serve as a joint preventive measure. When evaluating the question “does diabetes lead to cancer,” policymakers must consider economic, environmental, and social determinants that contribute to both epidemics. Collaborative public health strategies that treat metabolic and oncologic diseases as interconnected threats can better allocate resources and prioritize preventive care.
9. Could intermittent fasting or time-restricted eating reduce the likelihood that diabetes leads to cancer?
Intermittent fasting and time-restricted eating have shown promise in reducing insulin resistance and systemic inflammation, two key factors in the diabetes cancer connection. By improving insulin sensitivity and reducing circulating glucose and insulin levels, these dietary patterns may lower the biological drivers of tumor growth in diabetic individuals. Some studies also suggest that fasting induces cellular repair mechanisms like autophagy, which can remove damaged or precancerous cells. While more research is needed to confirm these benefits specifically in the context of diabetes and cancer, early findings are encouraging. If properly implemented under medical guidance, these strategies may serve as adjunctive tools to reduce the likelihood that diabetes leads to cancer.
10. What future technologies or innovations are being developed to better predict diabetes and cancer overlap?
Several emerging technologies aim to refine our ability to predict the overlap between diabetes and cancer. Artificial intelligence and machine learning algorithms are being trained on massive datasets to identify patterns in blood markers, genetic profiles, and imaging scans that may signal dual disease risk. Liquid biopsy technologies, which analyze circulating tumor DNA, are under investigation for their ability to detect early malignancies in diabetic individuals, particularly in cases of suspected diabetes and pancreatic cancer. Additionally, multi-omics platforms—integrating genomic, proteomic, and metabolomic data—offer holistic insights into the shared biological networks that underpin both diseases. These innovations hold the potential to transform how we answer the question “can diabetes lead to cancer” from a general inquiry into a personalized, actionable diagnosis.
Conclusion: Rethinking Chronic Disease Through a Unified Lens
So, can diabetes lead to cancer? While causality is complex and multifaceted, the scientific consensus increasingly affirms that diabetes—particularly type 2 diabetes—elevates the risk of developing certain cancers. From insulin signaling and chronic inflammation to oxidative stress and metabolic reprogramming, the biological overlap between diabetes and cancer is substantial. Epidemiological data, laboratory research, and clinical observations all converge on the insight that these two seemingly distinct diseases may, in fact, be deeply interwoven manifestations of systemic dysfunction.
Importantly, this understanding calls for a paradigm shift in how we approach chronic disease. Instead of treating diabetes and cancer as isolated conditions, healthcare systems must adopt integrated prevention strategies that address shared risk factors and underlying metabolic imbalances. Empowering patients with knowledge about how their metabolic health influences cancer risk can lead to more proactive engagement in lifestyle modification and routine screening.
Ultimately, the link between diabetes and cancer serves as a compelling reminder that health is holistic. By confronting metabolic disease with the same urgency and sophistication that we bring to oncology, we may not only improve outcomes for those with diabetes but also reduce the burden of cancer in the population at large. Continued research, personalized care, and comprehensive public health efforts will be essential in navigating this intricate intersection of chronic disease.
Further Reading:
The role of dietary sugars in cancer risk: A comprehensive review of current evidence
Diabetes and cancer: What you should know
Disclaimer
The information contained in this article is provided for general informational purposes only and is not intended to serve as medical, legal, or professional advice. While MedNewsPedia strives to present accurate, up-to-date, and reliable content, no warranty or guarantee, expressed or implied, is made regarding the completeness, accuracy, or adequacy of the information provided. Readers are strongly advised to seek the guidance of a qualified healthcare provider or other relevant professionals before acting on any information contained in this article. MedNewsPedia, its authors, editors, and contributors expressly disclaim any liability for any damages, losses, or consequences arising directly or indirectly from the use, interpretation, or reliance on any information presented herein. The views and opinions expressed in this article are those of the author(s) and do not necessarily reflect the official policies or positions of MedNewsPedia.