Maintaining the body’s internal environment in a narrow pH range is essential for survival. This delicate balance ensures that enzymatic activities, cellular metabolism, and physiological functions continue without disruption. While various buffering systems contribute to this equilibrium, proteins emerge as some of the most crucial components. Their unique structural properties and dynamic interactions allow them to serve as versatile regulators of acid-base homeostasis. Understanding how proteins function in this capacity not only illuminates the biochemical sophistication of the human body but also reveals their significance in dietary and clinical contexts. The exploration of how proteins regulate pH balance in the body bridges nutritional science with molecular physiology, offering insight into disease prevention, dietary planning, and therapeutic strategies.
You may also like: Macronutrients vs Micronutrients: What the Simple Definition of Macronutrients Reveals About Your Diet and Health
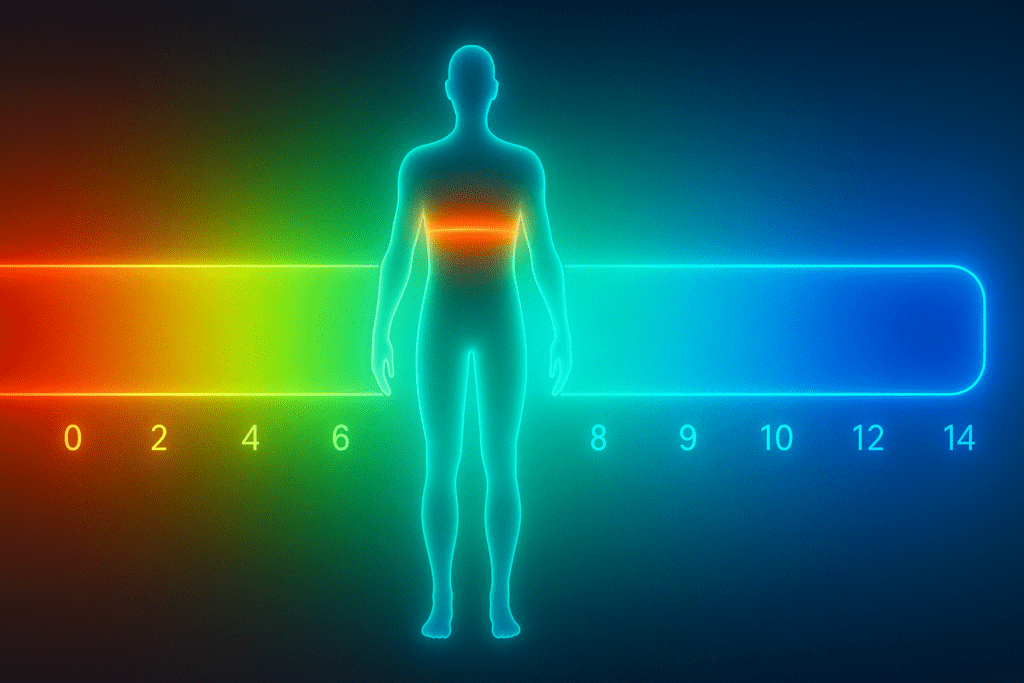
Understanding pH Balance and Why It Matters
To appreciate the role of proteins in maintaining pH stability, one must first understand what pH is. The term pH represents the concentration of hydrogen ions (H+) in a solution and is measured on a scale from 0 (highly acidic) to 14 (highly alkaline), with 7 being neutral. In humans, blood pH is normally regulated within a tight range of 7.35 to 7.45. Even slight deviations from this range can have profound consequences. A drop below 7.35 results in acidosis, while a rise above 7.45 leads to alkalosis. Both conditions can be life-threatening if left unchecked.
Various systems work in tandem to stabilize pH. These include the respiratory system, renal system, and a trio of buffer systems: bicarbonate, phosphate, and protein. Among these, the protein buffer system is the most abundant and versatile, functioning both intracellularly and extracellularly. But where do proteins regulate pH in the body, and how do they accomplish this intricate task? The answer lies in their molecular composition and interaction with hydrogen and hydroxide ions.
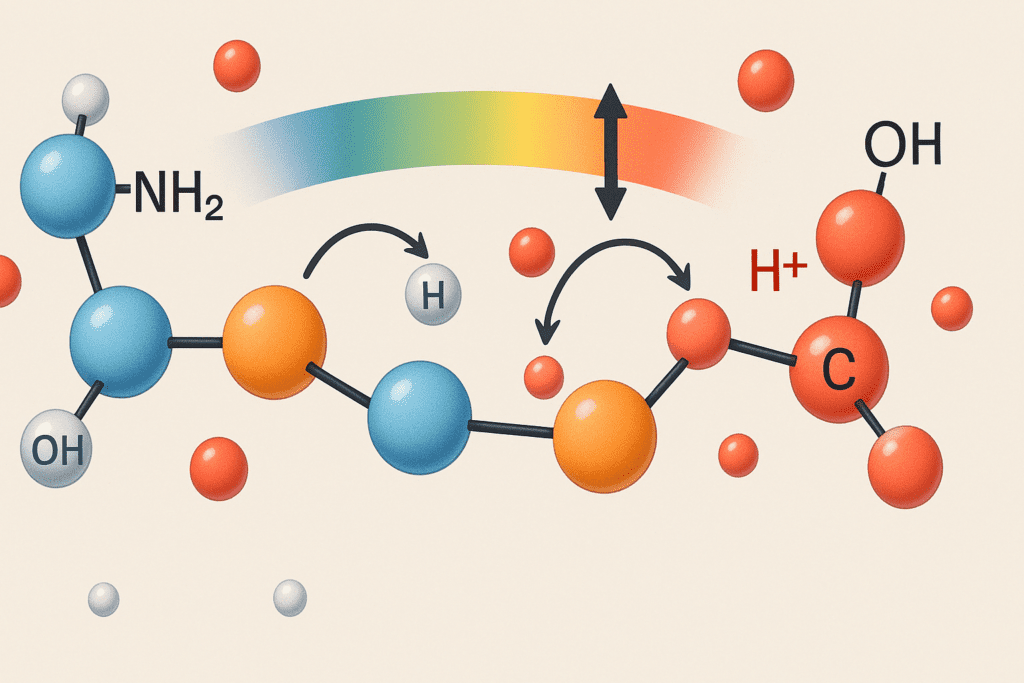
The Chemistry of Proteins as Buffers
Proteins are made up of long chains of amino acids, each containing both an amino group (NH2) and a carboxyl group (COOH). These functional groups grant proteins the ability to act as amphoteric molecules—substances that can donate or accept protons depending on the surrounding pH. When an environment becomes too acidic, proteins can absorb excess hydrogen ions through their amino groups. Conversely, when the environment is too basic, they can donate hydrogen ions from their carboxyl groups. This dual capability is at the heart of how proteins maintain pH balance in the body.
Moreover, the side chains (R groups) of certain amino acids also participate in buffering. For instance, the imidazole ring in histidine has a pKa near physiological pH, making it especially effective in buffering small changes. This characteristic is one reason why proteins containing histidine residues play a particularly significant role in acid-base regulation. The cumulative effect of these functional groups enables proteins to act rapidly and effectively, preventing dangerous swings in pH that could impair enzyme function or cellular integrity.
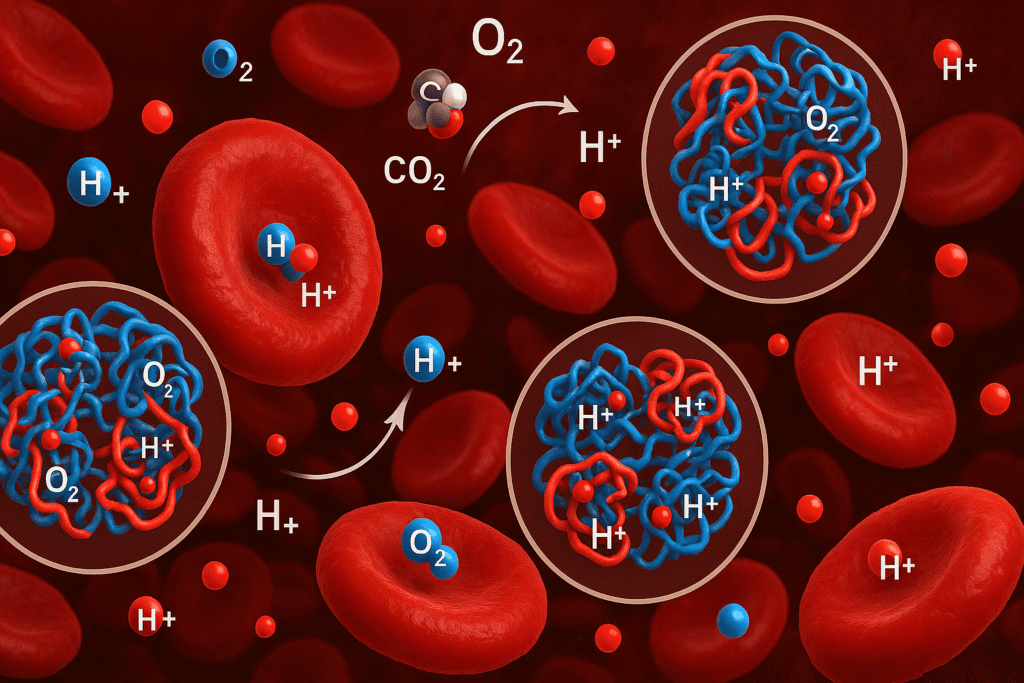
Hemoglobin: A Specialized Protein for Blood pH Regulation
One of the most extensively studied examples of protein-mediated pH regulation is hemoglobin, the oxygen-carrying protein found in red blood cells. Hemoglobin doesn’t just transport oxygen; it also acts as a buffer by binding to hydrogen ions. When carbon dioxide (CO2) enters red blood cells, it is converted to carbonic acid, which dissociates into bicarbonate and hydrogen ions. Hemoglobin binds many of these hydrogen ions, preventing a drop in pH.
This buffering action is critical in tissues with high metabolic activity, where CO2 production is elevated. By sequestering hydrogen ions, hemoglobin helps stabilize blood pH, even in conditions that would otherwise be strongly acidic. This function exemplifies how proteins regulate pH balance in highly dynamic physiological settings. The Bohr effect further illustrates this process, showing how hemoglobin’s oxygen affinity is influenced by pH and CO2 concentration, thereby linking respiratory gas exchange with acid-base regulation.
Plasma Proteins and Systemic Buffering
While hemoglobin acts within red blood cells, other proteins operate in the plasma to maintain pH balance. Chief among these is albumin, the most abundant protein in the bloodstream. Albumin contains multiple amino acid residues capable of binding hydrogen ions, thereby functioning as an extracellular buffer. Through this mechanism, it prevents sudden shifts in blood pH that could compromise cardiovascular stability or neurological function.
In addition to albumin, globulins and fibrinogen also contribute to systemic buffering. These proteins not only support immune function and clotting but also provide a supplementary means of acid-base regulation. Their collective presence in plasma ensures that the extracellular fluid remains within the optimal pH range. Understanding where proteins regulate pH in the body requires a holistic view, recognizing that they work in both circulating blood and within cellular compartments to guard against acidosis or alkalosis.
Intracellular Proteins and pH Homeostasis
Within cells, proteins play an equally vital role in pH regulation. The cytoplasm contains a dense matrix of structural and enzymatic proteins, many of which contain functional groups capable of buffering. As metabolic reactions proceed and generate acidic or basic byproducts, intracellular proteins absorb or donate hydrogen ions to maintain equilibrium.
This capacity becomes especially important in active tissues such as skeletal muscle, where intense activity leads to the production of lactic acid. Proteins within these cells help prevent local acidosis, which would otherwise impair muscle function and lead to fatigue. This illustrates how protein-mediated buffering supports not only biochemical processes but also physical performance. In this context, how proteins maintain pH balance in the body becomes not just a question of molecular chemistry but also one of functional physiology.
Dietary Protein and Acid-Base Interactions
Beyond endogenous protein function, dietary intake also influences pH regulation. High-protein diets, particularly those rich in animal proteins, are associated with increased acid load due to the metabolism of sulfur-containing amino acids like methionine and cysteine. These amino acids yield sulfuric acid, a strong acid that must be neutralized to maintain homeostasis.
The body responds by utilizing buffer systems, including protein buffers, to counteract this acid load. Over time, excessive dietary acid may place stress on the kidneys, which are responsible for excreting hydrogen ions and reabsorbing bicarbonate. However, moderate protein intake as part of a balanced diet supports both muscle maintenance and pH buffering. Thus, how protein keeps pH balanced extends to dietary choices and long-term nutritional strategies.
Research suggests that plant-based proteins may have a more neutral or even alkalizing effect, thanks to their accompanying mineral content, particularly potassium and magnesium. These minerals help support bicarbonate buffering and may reduce the net acid load. Therefore, understanding how proteins regulate pH balance involves examining both the biochemical role of endogenous proteins and the metabolic consequences of dietary intake.
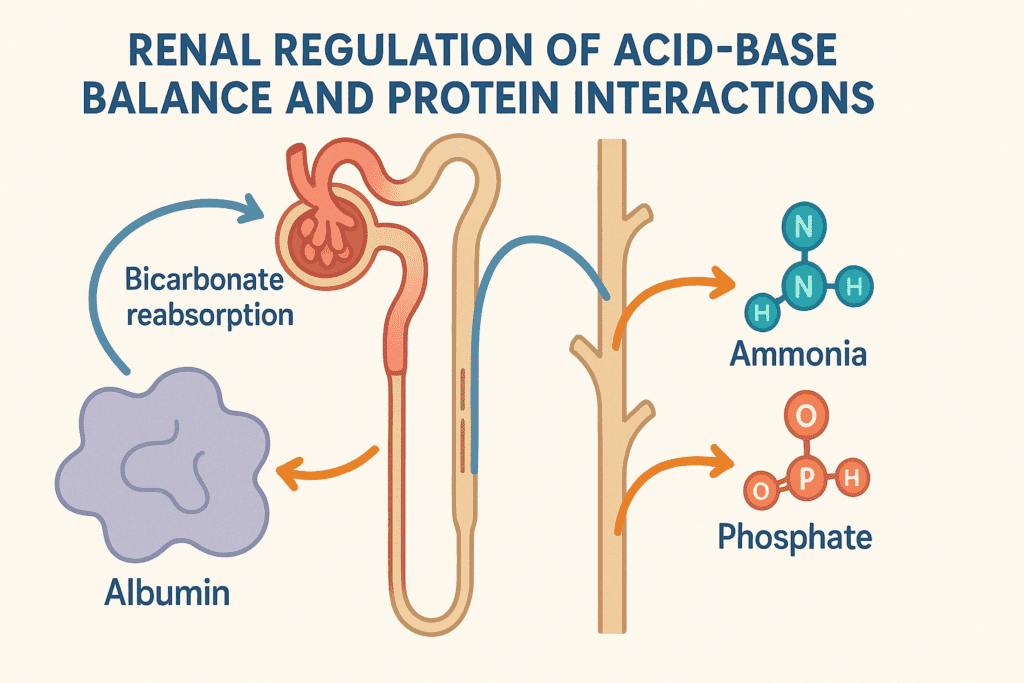
Renal Regulation of Acid-Base Balance and Protein Interactions
The kidneys are central to long-term pH regulation, and proteins play an indirect but crucial role in supporting renal function. As proteins are metabolized, they produce nitrogenous and sulfuric wastes, which must be excreted. This excretion requires buffering within the kidney tubules, a process aided by proteins such as ammonia and phosphate buffers.
Additionally, filtered plasma proteins influence the reabsorption of bicarbonate and secretion of hydrogen ions in the nephron. In chronic kidney disease, impaired excretion of acids can lead to metabolic acidosis, a condition where understanding how proteins regulate pH balance becomes even more relevant. Ensuring adequate dietary protein while not overwhelming the kidney’s excretory capacity becomes a delicate balance in these clinical scenarios.
Emerging studies have also highlighted how muscle protein breakdown in catabolic states can release amino acids that act as precursors for bicarbonate synthesis in the liver. This interplay shows the systemic nature of protein function in maintaining acid-base equilibrium and opens avenues for therapeutic interventions in acid-base disorders.
The Respiratory System and Protein Collaboration
Proteins do not operate in isolation. Their function in pH balance is tightly integrated with the respiratory system. As CO2 levels rise in the bloodstream, they increase hydrogen ion concentration, lowering pH. Proteins like hemoglobin respond by binding hydrogen ions, mitigating the pH shift.
This mechanism is bidirectional. When a person hyperventilates and expels CO2 rapidly, it can cause respiratory alkalosis. Proteins again help by releasing bound hydrogen ions to stabilize the alkalinity. This example illustrates how proteins, respiration, and renal function form a triad of interdependent systems to maintain acid-base homeostasis.
Therefore, where proteins regulate pH in the body spans multiple domains, from red blood cells to plasma, cells, and kidneys. Each interaction reflects an adaptive, responsive system fine-tuned through evolution to ensure survival in the face of varying internal and external challenges.
Protein Deficiency and Its Impact on pH Regulation
Protein malnutrition poses another layer of complexity. Without sufficient protein intake, the body’s buffering capacity diminishes. Low albumin levels, commonly seen in conditions like liver disease or chronic inflammation, reduce the blood’s ability to neutralize acids. Additionally, muscle wasting depletes intracellular protein stores that would otherwise assist in local pH regulation.
This can lead to a cascade of metabolic imbalances. In hospitalized patients, protein-energy malnutrition is a known risk factor for acidosis, especially when compounded by infection or renal impairment. Understanding how protein maintains pH balance in the body becomes essential not only in healthy individuals but also in the critically ill.
Nutritionists and clinicians must therefore consider protein adequacy not merely for growth and repair but also for its protective role in buffering and acid-base regulation. The goal is not only to prevent deficiency but also to optimize protein quality and quantity to meet the body’s buffering needs.
Clinical Applications and Therapeutic Implications
Recognizing how proteins regulate pH balance has far-reaching clinical implications. In patients with sepsis, trauma, or surgery, protein catabolism accelerates, reducing buffering capacity. Early nutritional intervention can help mitigate acid-base disturbances. Moreover, in conditions like diabetic ketoacidosis or chronic obstructive pulmonary disease (COPD), where acid-base imbalance is common, protein intake must be tailored to support both metabolic demand and buffering function.
Emerging therapies targeting protein interactions in acid-base balance are also on the horizon. Protein-based biosensors, synthetic buffers, and engineered peptides are being developed to modulate pH in clinical settings. These innovations reflect the growing appreciation of protein’s multifaceted role in homeostasis and the potential for leveraging this knowledge in personalized medicine.
From a public health perspective, educating the population on how protein keeps pH balanced can foster better dietary habits, especially in aging populations or those with chronic illness. Encouraging adequate, high-quality protein intake supports not only muscle health but also the body’s innate buffering systems, reinforcing wellness at the molecular level.
Frequently Asked Questions (FAQ): How Do Proteins Regulate pH Balance in the Body?
1. Can certain health conditions alter how proteins regulate pH balance in the body? Yes, chronic health conditions like kidney disease, liver dysfunction, and uncontrolled diabetes can significantly impair how proteins regulate pH balance. In kidney disease, the body struggles to excrete acidic metabolic waste, increasing reliance on protein-based buffers. Liver dysfunction can affect albumin production, compromising one of the major plasma proteins that help maintain pH. In such cases, even minor metabolic changes may trigger acidosis or alkalosis, highlighting the need for medical strategies that support protein function. Understanding where proteins regulate pH in the body becomes even more vital in these pathological scenarios.
2. How does protein intake affect the body’s ability to maintain pH balance over time? Protein intake has a nuanced impact on acid-base balance, especially when it comes to long-term dietary habits. Diets high in sulfur-containing amino acids from red meats may increase the acid load, while plant-based proteins often exert a more alkalizing effect. How does protein maintain pH balance in the body in this context? It depends on the body’s ability to metabolize and neutralize acidic byproducts using available buffering systems. Adequate hydration and mineral intake, especially potassium and magnesium, can enhance the body’s buffering response alongside proteins. Monitoring protein sources and combining them with alkaline-supporting foods is key for pH stability.
3. Are there differences in how animal and plant proteins regulate pH in the body? Yes, there are significant differences in how animal and plant proteins contribute to pH regulation. Animal proteins generally contain more sulfur-based amino acids, which produce more acidic waste during metabolism. In contrast, plant proteins are often accompanied by minerals that support alkalinity, such as calcium, magnesium, and potassium. While both sources provide the amino acids needed for buffering, the overall dietary pattern influences how proteins keep pH balanced. Diversifying protein sources can enhance how proteins regulate pH balance by balancing acid-forming and base-forming nutrients.
4. Where do proteins regulate pH in the body beyond the bloodstream? Although much focus is placed on blood pH, proteins regulate pH in numerous other areas including muscle tissue, the gastrointestinal tract, cerebrospinal fluid, and even within organelles like lysosomes. For example, intracellular proteins buffer acids in metabolically active tissues like the brain and skeletal muscle, where fluctuations in hydrogen ion concentration are common. Digestive enzymes, which are themselves proteins, also function optimally within specific pH ranges and may contribute indirectly to pH regulation by influencing gastrointestinal environment. Recognizing where proteins regulate pH in the body beyond systemic circulation offers a deeper understanding of their all-encompassing influence.
5. What role do proteins play in helping the body adapt to pH stress from high-intensity exercise? During high-intensity physical exertion, lactic acid accumulation threatens to disrupt pH balance in muscles and blood. Proteins within muscle cells help buffer this acid surge, allowing for prolonged physical activity and quicker recovery. One example is the role of carnosine, a dipeptide composed of beta-alanine and histidine, which acts as an efficient hydrogen ion buffer in muscle tissue. How does protein maintain pH balance in the body in this scenario? Through both structural proteins that act as pH reservoirs and specific buffer molecules that stabilize intracellular environments. Athletes often leverage nutritional strategies to support these buffering mechanisms.
6. Are there any symptoms that indicate protein-related pH buffering may be failing? Yes, when protein-based pH regulation begins to falter, the body may show early signs of imbalance such as muscle cramps, fatigue, confusion, or abnormal breathing patterns. These symptoms often emerge when the buffering systems are overwhelmed, such as during renal failure or metabolic disorders. In some cases, low serum albumin can serve as a biomarker that protein buffering capacity is compromised. Knowing how proteins regulate pH balance can guide both preventive care and early interventions in clinical settings. Symptom recognition can be especially important in vulnerable populations like the elderly or critically ill.
7. How does aging affect protein-mediated pH regulation in the body? With age, both protein synthesis and renal efficiency decline, which can impair how proteins regulate pH balance. Muscle mass diminishes, reducing the reservoir of intracellular proteins available for buffering. Simultaneously, albumin levels may decrease, weakening extracellular pH regulation. This dual impact makes older adults more susceptible to pH imbalances, especially when under stress from illness or medication. Understanding how protein keeps pH balanced throughout life underscores the importance of maintaining muscle health and adequate nutrition into older age.
8. Do protein supplements play a role in maintaining pH balance, especially for those with dietary limitations? Protein supplements can support pH balance, particularly for individuals with reduced food intake, restricted diets, or increased metabolic demands. However, the type of protein matters—whey protein, for example, may have a more acidogenic effect, while plant-based powders could offer a more neutral or slightly alkaline influence. How does protein keep pH balanced when supplemented? It provides essential amino acids necessary for endogenous buffer protein synthesis, such as albumin and enzymes involved in acid-base homeostasis. Selecting high-quality, appropriately dosed supplements can reinforce how proteins maintain pH balance in the body without overburdening metabolic pathways.
9. Can environmental or lifestyle factors influence how proteins regulate pH balance in the body? Absolutely. Chronic stress, sleep deprivation, poor hydration, and exposure to environmental toxins can all disrupt cellular metabolism, thereby increasing the production of acidic byproducts. Proteins then face increased demand as buffers, and when this demand exceeds capacity, pH imbalances may occur. How do proteins regulate pH balance under these circumstances? By adapting dynamically to metabolic shifts—but only to the extent that adequate protein and nutrient reserves are available. Integrating mindfulness, adequate rest, and hydration supports the broader framework in which proteins help regulate internal chemistry.
10. What are emerging research trends on how proteins regulate pH balance in the body? Recent studies are exploring how synthetic peptides and engineered protein molecules might enhance or mimic natural buffering systems. Scientists are also investigating the role of proteins in pH-sensitive drug delivery, using their buffering capacity to trigger release mechanisms in targeted therapies. Another area of interest is how gut microbiota influence systemic pH through protein metabolism, highlighting cross-talk between diet, microbiota, and host proteins. These advancements are reshaping how we understand where proteins regulate pH in the body—extending even into areas like biotechnology and precision medicine. The future holds potential for customized dietary and therapeutic approaches based on individual protein buffering profiles.
Conclusion: The Essential Role of Proteins in pH Stability and Long-Term Health
As this exploration has demonstrated, proteins are far more than the structural or enzymatic tools they are often portrayed to be. They are central players in one of the most critical aspects of human physiology—the regulation of pH. Whether through hemoglobin buffering hydrogen ions in red blood cells, albumin stabilizing plasma pH, or intracellular proteins guarding against metabolic acidosis, the body relies heavily on these macromolecules to stay within a life-sustaining pH range. Understanding how proteins regulate pH balance not only deepens our appreciation of molecular biology but also has practical implications for nutrition, clinical care, and disease prevention.
Where do proteins regulate pH in the body? The answer spans the bloodstream, cellular interior, diet, and kidneys—a network of finely tuned processes working in synchrony. How does protein keep pH balanced? Through chemical interactions, physiological coordination, and metabolic resilience. How does protein maintain pH balance in the body? By adapting to stressors, responding to dietary inputs, and participating in homeostatic feedback loops. And fundamentally, how do proteins regulate pH balance? They do so by being at the right place, at the right time, with the right biochemical properties.
For those seeking to optimize wellness, these insights affirm the importance of dietary choices, protein adequacy, and medical interventions that support acid-base balance. In this way, proteins act not only as building blocks of life but also as guardians of its fragile chemical harmony.
Further Reading:
On the pH-optimum of activity and stability of proteins
Understanding pH Balance in the Body