Introduction: Exploring the Power and Precision of Proton Therapy in Modern Oncology
In the ever-evolving landscape of cancer treatment, proton therapy has emerged as one of the most promising and sophisticated radiation technologies available. Distinguished by its unparalleled precision and reduced side effects, proton therapy has shifted the paradigm of care for patients with tumors located near vital structures or in pediatric populations. Unlike conventional X-ray radiation, proton therapy uses charged particles—protons—that can be controlled to deposit their energy directly in the tumor, sparing surrounding healthy tissues. This capability has spurred interest across the medical community and driven technological innovation in proton beam therapy machines, accelerators, and treatment planning systems.
You may also like: Cancer Research Breakthroughs: How Modern Advancements Are Transforming Treatment
As oncologists and medical physicists continue to explore its full potential, understanding the types of proton therapy machines and the complex proton beam therapy equipment used in these treatments is more important than ever. Behind every successful proton therapy treatment lies a highly coordinated interplay between advanced proton radiotherapy accelerators, gantries, and sophisticated imaging systems. These technologies are not only redefining cancer care but also inspiring a global race to improve access, precision, and affordability. From proton therapy accelerators to the design and layout of the proton beam accelerator room, every detail contributes to the remarkable effectiveness of this therapy.
The Science Behind Proton Therapy: A Breakthrough in Radiation Oncology
At the heart of proton therapy lies a distinctive physical principle: the Bragg peak. Protons release most of their energy at a specific depth before coming to a stop, allowing clinicians to precisely target tumors while minimizing exposure to surrounding tissues. This contrasts sharply with conventional X-ray radiation, which deposits energy throughout its path, affecting both cancerous and healthy tissues.
Proton therapy accelerators, essential to the function of these machines, energize protons to high velocities for targeted treatment. Coupled with sophisticated scanning magnets and tumor-tracking systems, these accelerators enable dynamic adjustments to beam shape and intensity. As adaptive therapy methods advance, clinicians can alter treatment plans in real-time, improving precision when tumors shift or change in size. The convergence of biology, physics, and engineering exemplifies how modern proton therapy machines push the boundaries of personalized medicine.

Inside the Proton Beam Accelerator Room: Engineering Marvels Behind the Treatment
The proton beam accelerator room represents the culmination of decades of research, development, and engineering excellence. Far from a simple treatment chamber, this space houses the core components that deliver life-saving precision therapy. Most rooms feature a large gantry system—a rotating structure that positions the proton beam from multiple angles—allowing clinicians to optimize dose delivery based on tumor location and patient anatomy.
Accelerators used in these rooms are either cyclotrons or synchrotrons. Cyclotrons provide a continuous stream of protons at a fixed energy, while synchrotrons can vary energy output, enhancing flexibility in treatment. The choice of accelerator impacts not only the treatment strategy but also the size and infrastructure of the facility. Newer compact proton therapy systems are redefining what the proton beam accelerator room can look like, offering smaller, cost-effective solutions without sacrificing clinical quality.
Advanced control systems within the room ensure the proton beam therapy machine functions with pinpoint accuracy. Sophisticated software regulates beam energy, monitors patient positioning, and communicates with imaging platforms in real time. The interplay between these technologies ensures that treatments are both safe and effective, delivering radiation within millimeters of precision. Beyond the physical equipment, environmental controls such as temperature, magnetic shielding, and vibration damping are also critical to maintaining optimal functionality of proton radiotherapy accelerators.
Global Expansion and Accessibility of Proton Therapy Centers
While once limited to only a few elite institutions, proton therapy is now experiencing global expansion. Centers are emerging across North America, Europe, and Asia, driven by increasing demand for precision oncology and investments in healthcare infrastructure. Public-private partnerships and international collaborations have facilitated knowledge exchange and reduced the entry barrier for new treatment facilities.
However, expanding access to proton therapy remains a complex endeavor. The initial cost of building a proton therapy center can exceed $100 million, posing challenges for health systems with limited budgets. In response, manufacturers are developing modular and single-room systems that make the technology more affordable and scalable. These innovations are critical for improving equity in cancer care and ensuring that patients in underserved regions can benefit from advanced treatments.
Global leaders like the United States, Japan, Germany, and South Korea continue to pioneer new clinical applications and conduct large-scale studies to validate outcomes. Emerging economies are also joining the movement, incorporating proton therapy into national cancer control strategies. This global commitment reflects a growing consensus: precision radiation is a cornerstone of the future of oncology.
Advancements in Proton Radiotherapy Accelerators and Imaging Integration
Technological breakthroughs in proton radiotherapy accelerators are redefining what is possible in cancer treatment. The evolution from bulky cyclotrons to superconducting synchrocyclotrons and linear accelerators has transformed the landscape of proton therapy. These next-generation machines offer enhanced energy modulation, smaller footprints, and faster treatment cycles.
Equally significant is the integration of imaging systems within the treatment process. In-room CT and MRI scanners, as well as surface-guided radiation therapy (SGRT), allow for real-time monitoring of tumor positioning. These tools ensure that radiation is delivered precisely where needed, even if the tumor moves between sessions. Some systems employ cone-beam CT or adaptive MRI, allowing daily treatment plans to be customized based on anatomical changes.
Such advancements support the vision of personalized medicine, where every aspect of treatment is tailored to the patient’s unique physiology and tumor characteristics. Artificial intelligence is increasingly playing a role, automating image analysis, predicting tumor response, and enhancing quality assurance. The fusion of hardware innovation with software intelligence marks a new era in cancer care, one that is more precise, efficient, and adaptable.
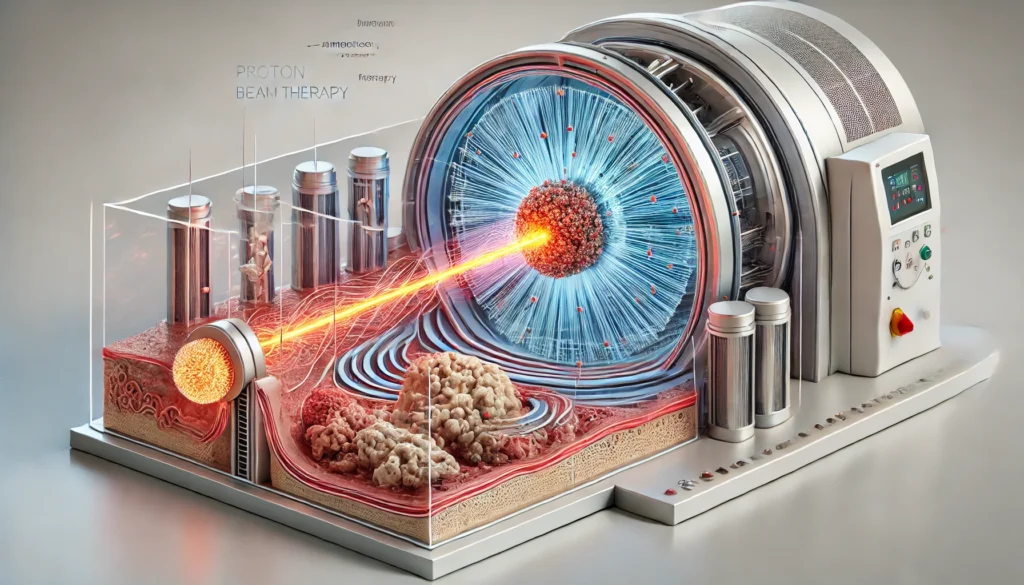
Patient Outcomes, Clinical Evidence, and Future Applications
The clinical evidence supporting proton therapy continues to grow, with numerous studies demonstrating reduced toxicity and improved quality of life for patients. In pediatric oncology, proton therapy has become a preferred modality for treating brain tumors, sarcomas, and other solid malignancies. Adult patients with complex head and neck, spinal, liver, and lung cancers also benefit significantly, particularly when tumors are located near critical structures.
Recent research highlights the potential of proton therapy in reirradiation—treating patients who have already received radiation in the past. This application is particularly valuable for recurrent cancers, where conventional therapy may not be viable due to dose limits. The precision of proton beam therapy equipment allows clinicians to retreat these areas safely, extending survival and improving outcomes.
Ongoing clinical trials are exploring proton therapy in new indications, including breast cancer, pancreatic cancer, and metastatic disease. These studies aim to solidify the role of proton therapy across the cancer spectrum, ensuring that evidence-based medicine guides future adoption. As data accumulates and technology advances, proton therapy is expected to become more cost-effective, making it a standard component of comprehensive cancer care.
Frequently Asked Questions: Advanced Insights into Proton Therapy Machines and Accelerators
1. How do the types of proton therapy machines influence treatment planning strategies?
The types of proton therapy machines used at a treatment center significantly influence how radiation is planned and delivered. Cyclotrons produce a constant proton beam at a fixed energy, requiring external devices to modulate energy levels, while synchrotrons offer adjustable beam energies at the source, which improves dose conformity in complex tumor shapes. These distinctions affect the degree of flexibility clinicians have when tailoring treatment to patient-specific tumor geometry. For example, certain brain and spine tumors may benefit more from synchrotron-based machines due to their nuanced energy control, especially when adjacent to critical organs. Choosing between these types of proton therapy machines can also impact scheduling efficiency, as synchrotrons may take longer between energy shifts, while cyclotrons offer faster treatment throughput.
2. What innovations are emerging in proton beam therapy machine design?
Recent innovations in proton beam therapy machine design are focused on reducing system size while maintaining precision and performance. Companies are developing compact, single-room units that use superconducting synchrocyclotrons, which provide the same clinical benefits with a smaller footprint. These machines can be installed in existing hospital spaces, unlike traditional systems that require dedicated buildings. Additionally, integration with real-time imaging and artificial intelligence algorithms is becoming standard, allowing machines to automatically adjust beam parameters based on tumor movement. These advancements in proton beam therapy machines aim to make proton therapy more accessible and cost-effective for hospitals worldwide.
3. How does proton beam therapy equipment enhance reirradiation protocols?
Reirradiation refers to delivering radiation to a site that has previously been treated, and proton beam therapy equipment is uniquely suited for this challenging scenario. Because protons deposit their maximum energy at a precise depth, they reduce exposure to tissues that may have already received significant radiation doses. This is critical in cases such as recurrent head and neck cancers, where surrounding structures have limited tolerance. Advanced imaging software integrated with proton beam therapy equipment can map dose accumulation over time and guide re-treatment planning. As a result, patients benefit from an extended therapeutic window and fewer cumulative side effects.
4. What practical considerations influence the design of a proton beam accelerator room?
Designing a proton beam accelerator room involves more than installing treatment hardware; it requires precision engineering and environmental control. Shielding must be optimized to protect staff from secondary radiation while maintaining patient comfort. Acoustic dampening, temperature regulation, and even humidity control are essential to keep proton therapy accelerators operating within optimal ranges. Emerging technologies also support modular room configurations that adapt to different types of proton therapy machines, allowing centers to scale or upgrade equipment with minimal renovation. These design decisions directly affect clinical efficiency, safety compliance, and long-term operational cost.
5. What distinguishes a proton therapy accelerator from conventional radiation accelerators?
Unlike conventional linear accelerators that produce X-rays, a proton therapy accelerator generates charged particles with mass, requiring more complex systems for beam generation and control. The presence of beam transport lines, energy selection systems, and higher radiation shielding sets proton therapy accelerators apart from their photon-based counterparts. These systems can also vary the beam’s depth and intensity dynamically, enabling more targeted treatment. Furthermore, the maintenance demands of a proton therapy accelerator are higher due to the precision components involved, including superconducting magnets and vacuum chambers. Despite these complexities, the clinical advantages in sparing healthy tissues and improving outcomes justify their growing adoption.
6. Can proton radiotherapy accelerators be used in mobile or transportable units?
While still experimental, there are ongoing efforts to miniaturize proton radiotherapy accelerators to the point where they could be incorporated into mobile or modular units. Superconducting magnet technology and compact linac designs are at the forefront of this innovation. The primary challenge lies in ensuring beam stability and safety in a transportable configuration, particularly in environments that lack permanent shielding. However, early prototypes suggest that regional cancer centers could one day share mobile proton units, dramatically expanding access. This vision represents a major shift from centralized care to more decentralized, community-based proton therapy solutions.
7. How do clinicians choose between different proton beam therapy accelerators?
Choosing the right proton beam therapy accelerator depends on several clinical, logistical, and economic factors. Institutions with high patient volumes and complex cases may prefer synchrotrons for their energy modulation capabilities, while centers prioritizing throughput and operational simplicity often select cyclotrons. Cost, space requirements, and integration with existing hospital infrastructure also influence decision-making. Some centers may invest in hybrid systems or customizable platforms that support future upgrades. Ultimately, the choice of a proton beam therapy accelerator is guided by both clinical strategy and long-term sustainability goals.
8. What are the future trends in proton beam accelerator room configuration?
The traditional proton beam accelerator room is evolving to accommodate digital workflows and more ergonomic designs. Future layouts will likely include integrated imaging stations, real-time motion tracking, and AI-driven adaptive planning interfaces. In-room displays, automated patient positioning systems, and voice-activated controls are also being tested to improve treatment precision and workflow efficiency. Efforts are underway to make proton beam accelerator rooms more accessible for pediatric patients by incorporating child-friendly designs and psychological support features. These advancements reflect a shift toward more patient-centered, technologically seamless environments.
9. How does maintenance of proton beam therapy equipment differ from other medical devices?
Maintaining proton beam therapy equipment involves specialized engineering support due to the system’s complexity and precision requirements. Routine calibration of beam alignment, energy modulation, and scanning accuracy is essential to ensure safety and efficacy. Unlike standard radiation machines, which may be serviced with general biomedical support, proton systems require dedicated physicists and manufacturer-trained technicians. Unplanned downtime can affect treatment schedules significantly, so many centers invest in redundant systems and predictive maintenance technologies. As these machines evolve, remote diagnostics and AI-based service alerts are becoming more prevalent to prevent disruptions.
10. What role do patient-reported outcomes play in optimizing proton therapy accelerator usage?
Patient-reported outcomes (PROs) offer critical insights into how individuals experience treatment with a proton therapy accelerator. These reports help clinicians fine-tune treatment parameters to reduce fatigue, skin reactions, or other side effects that may not be visible through imaging alone. Incorporating PROs into treatment planning allows for a more holistic, feedback-driven approach, particularly when using different types of proton therapy machines that vary in dose distribution. Long-term data on quality of life also inform future upgrades to proton radiotherapy accelerators, guiding technological innovation based on patient needs. As value-based care becomes a priority, leveraging PROs will be essential in evaluating the real-world effectiveness of proton therapy delivery platforms.

Conclusion: The Future of Cancer Treatment Through the Lens of Proton Beam Innovation
Proton therapy represents one of the most transformative advances in modern oncology. From the powerful proton therapy accelerator to the complexity of the proton beam accelerator room, each element is a testament to scientific ingenuity and a commitment to precision medicine. As the field continues to evolve, the types of proton therapy machines available will only grow more advanced, compact, and accessible, democratizing this life-saving technology across the globe.
The synergy between proton beam therapy machines, real-time imaging, artificial intelligence, and personalized treatment planning is revolutionizing how we approach cancer care. Patients are no longer limited to one-size-fits-all treatment; they now benefit from customized therapies that minimize harm and maximize outcomes. Institutions worldwide are embracing this innovation, not only for its clinical superiority but also for its potential to improve lives.
As we look ahead, the fusion of engineering, clinical research, and data science will propel proton therapy into an era where every cancer patient receives the most precise, compassionate, and effective care possible. In this future, guided by the continuing evolution of proton beam therapy equipment and proton radiotherapy accelerators, cancer treatment will no longer be defined by compromise, but by cure.
advanced radiation therapy, precision cancer treatment, pediatric oncology radiation, targeted radiation therapy, non-invasive cancer therapy, cancer treatment innovation, cutting-edge oncology technology, particle beam therapy, medical imaging in oncology, radiation dose modulation, cancer center technology, radiation oncology trends, tumor-specific radiation, next-generation radiation machines, image-guided therapy, adaptive radiation planning, oncology equipment innovations, high-precision radiation therapy, cancer treatment accelerators, compact radiation systems
Further Reading:
Proton Therapy: An Examination of Challenges and Future Directions
Three ways to make proton therapy affordable
Disclaimer
The information contained in this article is provided for general informational purposes only and is not intended to serve as medical, legal, or professional advice. While MedNewsPedia strives to present accurate, up-to-date, and reliable content, no warranty or guarantee, expressed or implied, is made regarding the completeness, accuracy, or adequacy of the information provided. Readers are strongly advised to seek the guidance of a qualified healthcare provider or other relevant professionals before acting on any information contained in this article. MedNewsPedia, its authors, editors, and contributors expressly disclaim any liability for any damages, losses, or consequences arising directly or indirectly from the use, interpretation, or reliance on any information presented herein. The views and opinions expressed in this article are those of the author(s) and do not necessarily reflect the official policies or positions of MedNewsPedia.